Novel Cyclic Lipopeptide Antibiotics: Effects of Acyl Chain Length and Position
- PMID: 32823798
- PMCID: PMC7461568
- DOI: 10.3390/ijms21165829
Novel Cyclic Lipopeptide Antibiotics: Effects of Acyl Chain Length and Position
Abstract
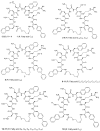
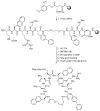
Multidrug-resistant bacteria are a global health problem. One of the last-resort antibiotics against Gram-negative bacteria is the cyclic lipopeptide colistin, displaying a flexible linker with a fatty acid moiety. The aim of the present project was to investigate the effect on antimicrobial activity of introducing fatty acid moieties of different lengths and in different positions in a cyclic peptide, S3(B), containing a flexible linker. The lipidated analogues of S3(B) were synthesized by 9-fluorenylmethoxycarbonyl (Fmoc) solid-phase peptide synthesis. Following assembly of the linear peptide by Fmoc solid-phase peptide synthesis, on-resin head-to-tail cyclization and fatty acid acylation were performed. The antimicrobial activity was determined against the ESKAPE pathogens, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Escherichia coli. Furthermore, hemolytic activity was determined against human erythrocytes. A total of 18 cyclic lipopeptides were synthesized and characterized. It was found that introduction of fatty acids in positions next to the flexible linker was more strongly linked to antimicrobial activity. The fatty acid length altered the overall hydrophobicity, which was the driving force for both high antimicrobial and hemolytic activity. Peptides became highly hemolytic when carbon-chain length exceeded 10 (i.e., C10), overlapping with the optimum for antimicrobial activity (i.e., C8-C12). The most promising candidate (C8)5 showed antimicrobial activity corresponding to that of S3(B), but with an improved hemolytic profile. Finally, (C8)5 was further investigated in a time-kill experiment.
Keywords: antimicrobial peptides; colistin; cyclic lipopeptides; fatty acid; hydrophobicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- WHO Critically Important Antimicrobials for Human Medicine. [(accessed on 6 August 2020)]; Available online: https://www.who.int/foodsafety/publications/antimicrobials-sixth/en/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

