Emerging Cancer Epigenetic Mechanisms Regulated by All-Trans Retinoic Acid
- PMID: 32823855
- PMCID: PMC7465226
- DOI: 10.3390/cancers12082275
Emerging Cancer Epigenetic Mechanisms Regulated by All-Trans Retinoic Acid
Abstract
All-trans retinoic acid (RA), which is the dietary bioactive derivative obtained from animal (retinol) and plant sources (beta-carotene), is a physiological lipid signal of both embryonic and postembryonic development. During pregnancy, either RA deficiency or an excessive RA intake is teratogenic. Too low or too high RA affects not only prenatal, but also postnatal, developmental processes such as myelopoiesis and mammary gland morphogenesis. In this review, we mostly focus on emerging RA-regulated epigenetic mechanisms involving RA receptor alpha (RARA) and Annexin A8 (ANXA8), which is a member of the Annexin family, as well as ANXA8 regulatory microRNAs (miRNAs). The first cancer showing ANXA8 upregulation was reported in acute promyelocytic leukemia (APL), which induces the differentiation arrest of promyelocytes due to defective RA signaling caused by RARA fusion genes as the PML-RARA gene. Over the years, ANXA8 has also been found to be upregulated in other cancers, even in the absence of RARA fusion genes. Mechanistic studies on human mammary cells and mammary glands of mice showed that ANXA8 upregulation is caused by genetic mutations affecting RARA functions. Although not all of the underlying mechanisms of ANXA8 upregulation have been elucidated, the interdependence of RA-RARA and ANXA8 seems to play a relevant role in some normal and tumorigenic settings.
Keywords: RA receptor α (RARA); all-trans retinoic acid (RA); annexins; cancer; microRNAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


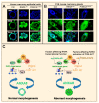
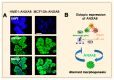

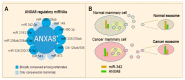
References
-
- Johnson E.J., Russell R.M. Beta-carotene. In: Coates P.M., Betz J.M., Blackman M.R., Cragg G.M., Levine M., Moss J., White J.D., editors. Encyclopedia of Dietary Supplements. 2nd ed. Informa Healthcare; London, UK: New York, NY, USA: 2010. pp. 115–120.
-
- Ross A., Vitamin A. Carotenoids. In: Shils M.S.M., Ross A., Caballero B., Cousins R., editors. Modern Nutrition in Health and Disease. 10th ed. Lippincott Williams & Wilkins; Baltimore, MD, USA: 2006. pp. 351–375.
-
- Ross C.A., Vitamin A. In: Encyclopedia of Dietary Supplements. 2nd ed. Coates P.M., Betz J.M., Blackman M.R., Cragg G.M., Levine M., Moss J., White J.D., editors. Informa Healthcare; London, UK: New York, NY, USA: 2010. pp. 778–791.
-
- Solomons N.W., Vitamin A. In: Present Knowledge in Nutrition. 9th ed. Bowman B., Russell R., editors. International Life Sciences Institute; Washington, DC, USA: 2006. pp. 157–183.
Publication types
LinkOut - more resources
Full Text Sources

