Melittin from Apis florea Venom as a Promising Therapeutic Agent for Skin Cancer Treatment
- PMID: 32823904
- PMCID: PMC7460526
- DOI: 10.3390/antibiotics9080517
Melittin from Apis florea Venom as a Promising Therapeutic Agent for Skin Cancer Treatment
Abstract
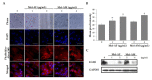
Melittin, a major component found in bee venom, is produced by the Apis species of the honey bee. In this study, the effect of melittin derived from Apis florea (Mel-AF), which is a wild honey bee species that is indigenous to Thailand, was investigated against human malignant melanoma (A375) cells. In this study, Mel-AF exhibited considerable potential in the anti-proliferative action of A375 cells. Subsequently, the cellular mechanism of Mel-AF that induced cell death was investigated in terms of apoptosis. As a result, gene and protein expression levels, which indicated the activation of cytochrome-c release and caspase-9 expression, eventually triggered the release of the caspase-3 executioner upon Mel-AF. We then determined that apoptosis-mediated cell death was carried out through the intrinsic mitochondrial pathway. Moreover, advanced abilities, including cell motility and invasion, were significantly suppressed. Mel-AF manipulated the actin arrangement via the trapping of stress fibers that were found underneath the membrane, which resulted in the defective actin cytoskeleton organization. Consequently, the expression of EGFR, a binding protein to F-actin, was also found to be suppressed. This outcome strongly supports the effects of Mel-AF in the inhibition of progressive malignant activity through the disruption of actin cytoskeleton-EGFR interaction and the EGFR signaling system. Thus, the findings of our current study indicate the potential usefulness of Mel-AF in cancer treatments as an apoptosis inducer and a potential actin-targeting agent.
Keywords: Apis florea; F-actin; apoptosis; epidermal growth factor receptor; malignant melanoma; melittin.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
A Comparative Study of Melittins from Apis florea and Apis mellifera as Cytotoxic Agents Against Non-Small Cell Lung Cancer (NSCLC) Cells and Their Combination with Gefitinib.Int J Mol Sci. 2025 Mar 11;26(6):2498. doi: 10.3390/ijms26062498. Int J Mol Sci. 2025. PMID: 40141140 Free PMC article.
-
Anti-Herpes Simplex Virus and Anti-Inflammatory Activities of the Melittin Peptides Derived from Apis mellifera and Apis florea Venom.Insects. 2024 Feb 4;15(2):109. doi: 10.3390/insects15020109. Insects. 2024. PMID: 38392528 Free PMC article.
-
Functional characterization of naturally occurring melittin peptide isoforms in two honey bee species, Apis mellifera and Apis cerana.Peptides. 2014 Mar;53:185-93. doi: 10.1016/j.peptides.2014.01.026. Epub 2014 Feb 8. Peptides. 2014. PMID: 24512991
-
Application of bee venom and its main constituent melittin for cancer treatment.Cancer Chemother Pharmacol. 2016 Dec;78(6):1113-1130. doi: 10.1007/s00280-016-3160-1. Epub 2016 Sep 27. Cancer Chemother Pharmacol. 2016. PMID: 27677623 Review.
-
Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy.Cancer Lett. 2017 Aug 28;402:16-31. doi: 10.1016/j.canlet.2017.05.010. Epub 2017 May 20. Cancer Lett. 2017. PMID: 28536009 Free PMC article. Review.
Cited by
-
Bee chitosan nanoparticles loaded with apitoxin as a novel approach to eradication of common human bacterial, fungal pathogens and treating cancer.Front Microbiol. 2024 Mar 15;15:1345478. doi: 10.3389/fmicb.2024.1345478. eCollection 2024. Front Microbiol. 2024. PMID: 38559346 Free PMC article.
-
A new therapeutic approach for bone metastasis in colorectal cancer: intratumoral melittin.J Venom Anim Toxins Incl Trop Dis. 2022 Mar 14;28:e20210067. doi: 10.1590/1678-9199-JVATITD-2021-0067. eCollection 2022. J Venom Anim Toxins Incl Trop Dis. 2022. PMID: 35321289 Free PMC article.
-
Hybrid bio-nanoporous peptide loaded-polymer platforms with anticancer and antibacterial activities.Nanoscale Adv. 2024 Feb 27;6(8):2038-2058. doi: 10.1039/d3na00947e. eCollection 2024 Apr 16. Nanoscale Adv. 2024. PMID: 38633049 Free PMC article.
-
Pharmacological effects and mechanisms of bee venom and its main components: Recent progress and perspective.Front Pharmacol. 2022 Sep 27;13:1001553. doi: 10.3389/fphar.2022.1001553. eCollection 2022. Front Pharmacol. 2022. PMID: 36238572 Free PMC article. Review.
-
Bioactive peptides from food science to pharmaceutical industries: Their mechanism of action, potential role in cancer treatment and available resources.Heliyon. 2024 Nov 22;10(23):e40563. doi: 10.1016/j.heliyon.2024.e40563. eCollection 2024 Dec 15. Heliyon. 2024. PMID: 39654719 Free PMC article. Review.
References
-
- Meng X.J., Ao H.F., Huang W.T., Chen F., Sun X.C., Wang J.J., Lui Z.F., Han W.W., Fry A.N., Wang D.H. Impact of different surgical and postoperative adjuvant treatment modalities on survival of sinonasal malignant melanoma. BMC Cancer. 2014;14:608. doi: 10.1186/1471-2407-14-608. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

