Pembrolizumab Activity in Recurrent High-Grade Gliomas with Partial or Complete Loss of Mismatch Repair Protein Expression: A Monocentric, Observational and Prospective Pilot Study
- PMID: 32823925
- PMCID: PMC7464918
- DOI: 10.3390/cancers12082283
Pembrolizumab Activity in Recurrent High-Grade Gliomas with Partial or Complete Loss of Mismatch Repair Protein Expression: A Monocentric, Observational and Prospective Pilot Study
Abstract
Introduction: Pembrolizumab demonstrated promising results in hypermutated tumors of diverse origin. Immunohistochemical loss of mismatch repair (MMR) proteins has been suggested as a surrogate of hypermutation in high-grade gliomas (HGG). We evaluated the efficacy and safety of pembrolizumab in relapsing HGGs with immunohistochemical loss of at least 1 MMR protein. Molecular biomarkers of pembrolizumab activity were also analyzed.
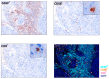
Methods: Consecutive patients with recurrent HGG and partial or complete loss of MMR protein expression were prospectively enrolled; they received pembrolizumab 200 mg once every 3 weeks until disease progression. The primary endpoint was disease control rate (DCR). Post hoc exploratory analyses included next-generation sequencing to assess tumor mutational burden (TMB), and immunostaining for CD8+ T-cells and CD68+ macrophages.
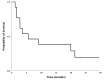
Results: Among 310 HGG patients screened, 13 cases with MMR loss were enrolled: eight glioblastoma, four anaplastic astrocytoma, and one anaplastic oligodendroglioma. Median age was 43 years. DCR was 31%: four patients had stable disease and no patient had complete or partial response. TMB ranged between 6.8 and 23.4 mutations/megabase. Neither TMB nor gene mutations, nor CD8+ T-cell and CD68+ macrophage content, were associated with pembrolizumab activity.
Conclusions: pembrolizumab showed no apparent benefit in these patients. No molecular biomarker was found to be associated with pembrolizumab activity.
Keywords: TMB; glioblastoma; high grade glioma; mismatch repair; pembrolizumab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Dolcetti R., Viel A., Doglioni C., Russo A., Guidoboni M., Capozzi E., Vecchiato N., Macri E., Fornasarig M., Boiocchi M. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am. J. Pathol. 1999;154:1805–1813. doi: 10.1016/S0002-9440(10)65436-3. - DOI - PMC - PubMed
-
- Luchini C., Bibeau F., Ligtenberg M.J.L., Singh N., Nottegar A., Bosse T., Miller R., Riaz N., Douillard J.-Y., Andre F., et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann. Oncol. 2019;30:1232–1243. doi: 10.1093/annonc/mdz116. - DOI - PubMed
-
- Hodges T.R., Ott M., Xiu J., Gatalica Z., Swensen J., Zhou S., Huse J.T., de Groot J., Li S., Overwijk W.W., et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: Implications for immune checkpoint immunotherapy. Neuro Oncol. 2017;19:1047–1057. doi: 10.1093/neuonc/nox026. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

