Lipid Metabolism and Cancer Immunotherapy: Immunosuppressive Myeloid Cells at the Crossroad
- PMID: 32823961
- PMCID: PMC7461616
- DOI: 10.3390/ijms21165845
Lipid Metabolism and Cancer Immunotherapy: Immunosuppressive Myeloid Cells at the Crossroad
Abstract
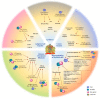
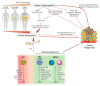
Cancer progression generates a chronic inflammatory state that dramatically influences hematopoiesis, originating different subsets of immune cells that can exert pro- or anti-tumor roles. Commitment towards one of these opposing phenotypes is driven by inflammatory and metabolic stimuli derived from the tumor-microenvironment (TME). Current immunotherapy protocols are based on the reprogramming of both specific and innate immune responses, in order to boost the intrinsic anti-tumoral activity of both compartments. Growing pre-clinical and clinical evidence highlights the key role of metabolism as a major influence on both immune and clinical responses of cancer patients. Indeed, nutrient competition (i.e., amino acids, glucose, fatty acids) between proliferating cancer cells and immune cells, together with inflammatory mediators, drastically affect the functionality of innate and adaptive immune cells, as well as their functional cross-talk. This review discusses new advances on the complex interplay between cancer-related inflammation, myeloid cell differentiation and lipid metabolism, highlighting the therapeutic potential of metabolic interventions as modulators of anticancer immune responses and catalysts of anticancer immunotherapy.
Keywords: cancer immunotherapy; cholesterol; fatty acids; lipid metabolism; myeloid-derived suppressor cells (MDSCs); obesity; tumor-associated macrophages (TAMs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

