Contribution of Predictive and Prognostic Biomarkers to Clinical Research on Chronic Kidney Disease
- PMID: 32823966
- PMCID: PMC7461617
- DOI: 10.3390/ijms21165846
Contribution of Predictive and Prognostic Biomarkers to Clinical Research on Chronic Kidney Disease
Abstract
Chronic kidney disease (CKD), defined as the presence of albuminuria and/or reduction in estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, is considered a growing public health problem, with its prevalence and incidence having almost doubled in the past three decades. The implementation of novel biomarkers in clinical practice is crucial, since it could allow earlier diagnosis and lead to an improvement in CKD outcomes. Nevertheless, a clear guidance on how to develop biomarkers in the setting of CKD is not yet available. The aim of this review is to report the framework for implementing biomarkers in observational and intervention studies. Biomarkers are classified as either prognostic or predictive; the first type is used to identify the likelihood of a patient to develop an endpoint regardless of treatment, whereas the second type is used to determine whether the patient is likely to benefit from a specific treatment. Many single assays and complex biomarkers were shown to improve the prediction of cardiovascular and kidney outcomes in CKD patients on top of the traditional risk factors. Biomarkers were also shown to improve clinical trial designs. Understanding the correct ways to validate and implement novel biomarkers in CKD will help to mitigate the global burden of CKD and to improve the individual prognosis of these high-risk patients.
Keywords: CKD; biomarkers; cardiovascular disease; end-stage kidney disease (ESKD); epidemiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
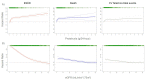
References
-
- FDA-NIH Biomarker Working Group . BEST (Biomarkers, Endpoints, and other Tools) Resource. Food and Drug Administration (US); Silver Spring, MD, USA: National Institutes of Health (US); Bethesda, MD, USA: 2020. [(accessed on 25 June 2020)]. Available online: www.ncbi.nlm.nih.gov/books/NBK326791. - PubMed
-
- Pepe M., Janes H. Methods for Evaluating Prediction Performance of Biomarkers and Tests. In: Lee M.L., Gail M., Pfeiffer R., Satten G., Cai T., Gandy A., editors. Risk Assessment and Evaluation of Predictions. Volume 215. Springer; New York, NY, USA: 2013. Lecture Notes in Statistics. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

