Hepatitis E Virus Mediates Renal Injury via the Interaction between the Immune Cells and Renal Epithelium
- PMID: 32824088
- PMCID: PMC7564770
- DOI: 10.3390/vaccines8030454
Hepatitis E Virus Mediates Renal Injury via the Interaction between the Immune Cells and Renal Epithelium
Abstract
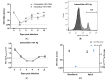
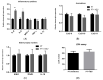
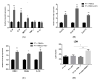
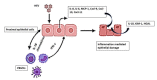
Renal disorders are associated with Hepatitis E virus (HEV) infection. Progression to end-stage renal disease and acute kidney injury are complications associated with HEV infection. The mechanisms by which HEV mediates the glomerular diseases remain unclear. CD10+/CD13+ primary proximal tubular (PT) epithelial cells, isolated from healthy donors, were infected with HEV. Inflammatory markers and kidney injury markers were assessed in the presence or absence of peripheral blood mononuclear cells (PBMCs) isolated from the same donors. HEV replicated efficiently in the PT cells as shown by the increase in HEV load over time and the expression of capsid Ag. In the absence of PBMCs, HEV was not nephrotoxic, with no direct effect on the transcription of chemokines (Cxcl-9, Cxcl-10, and Cxcl-11) nor the kidney injury markers (kidney injury molecule 1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), and interleukin 18 (lL-18)). While higher inflammatory responses, upregulation of chemokines and kidney injury markers expression, and signs of nephrotoxicity were recorded in HEV-infected PT cells cocultured with PBMCs. Interestingly, a significantly higher level of IFN-γ was released in the PBMCs-PT coculture compared to PT alone during HEV infection. In conclusion: The crosstalk between immune cells and renal epithelium and the signal axes IFN-γ/chemokines and IL-18 could be the immune-mediated mechanisms of HEV-induced renal disorder.
Keywords: HEV; IFN-γ; chemokines; immune-mediated; inflammatory cytokines; kidney injury; proximal tubular; renal disorder.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sayed I.M., Vercauteren K., Abdelwahab S.F., Meuleman P. The emergence of hepatitis E virus in Europe. Future Virol. 2015;10:763–778. doi: 10.2217/fvl.15.29. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

