Synthesis and Characterization of Oxidized Polysaccharides for In Situ Forming Hydrogels
- PMID: 32824101
- PMCID: PMC7464976
- DOI: 10.3390/biom10081185
Synthesis and Characterization of Oxidized Polysaccharides for In Situ Forming Hydrogels
Abstract
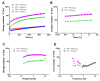
Polysaccharides are widely used as building blocks of scaffolds and hydrogels in tissue engineering, which may require their chemical modification to permit crosslinking. The goal of this study was to generate a library of oxidized alginate (oALG) and oxidized hyaluronic acid (oHA) that can be used for in situ gelling hydrogels by covalent reaction between aldehyde groups of the oxidized polysaccharides (oPS) and amino groups of carboxymethyl chitosan (CMC) through imine bond formation. Here, we studied the effect of sodium periodate concentration and reaction time on aldehyde content, molecular weight of derivatives and cytotoxicity of oPS towards 3T3-L1 fibroblasts. It was found that the molecular weights of all oPs decreased with oxidation and that the degree of oxidation was generally higher in oHA than in oALG. Studies showed that only oPs with an oxidation degree above 25% were cytotoxic. Initial studies were also done on the crosslinking of oPs with CMC showing with rheometry that rather soft gels were formed from higher oxidized oPs possessing a moderate cytotoxicity. The results of this study indicate the potential of oALG and oHA for use as in situ gelling hydrogels or inks in bioprinting for application in tissue engineering and controlled release.
Keywords: alginate; cytotoxicity; fibroblasts; hyaluronic acid; hydrogels; in situ gelling; oxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Domingues R.M., Silva M., Gershovich P., Betta S., Babo P., Caridade S.G., Mano J.o.F., Motta A., Reis R.L., Gomes M.E. Development of injectable hyaluronic acid/cellulose nanocrystals bionanocomposite hydrogels for tissue engineering applications. Bioconjugate Chem. 2015;26:1571–1581. doi: 10.1021/acs.bioconjchem.5b00209. - DOI - PubMed
-
- Pop-Georgievski O., Zimmermann R., Kotelnikov I., Proks V., Romeis D., Kučka J., Caspari A., Rypáček F.E., Werner C. Impact of bioactive peptide motifs on molecular structure, charging, and nonfouling properties of poly (ethylene oxide) brushes. Langmuir. 2018;34:6010–6020. doi: 10.1021/acs.langmuir.8b00441. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

