Myosin XVI in the Nervous System
- PMID: 32824179
- PMCID: PMC7464383
- DOI: 10.3390/cells9081903
Myosin XVI in the Nervous System
Abstract
The myosin family is a large inventory of actin-associated motor proteins that participate in a diverse array of cellular functions. Several myosin classes are expressed in neural cells and play important roles in neural functioning. A recently discovered member of the myosin superfamily, the vertebrate-specific myosin XVI (Myo16) class is expressed predominantly in neural tissues and appears to be involved in the development and proper functioning of the nervous system. Accordingly, the alterations of MYO16 has been linked to neurological disorders. Although the role of Myo16 as a generic actin-associated motor is still enigmatic, the N-, and C-terminal extensions that flank the motor domain seem to confer unique structural features and versatile interactions to the protein. Recent biochemical and physiological examinations portray Myo16 as a signal transduction element that integrates cell signaling pathways to actin cytoskeleton reorganization. This review discusses the current knowledge of the structure-function relation of Myo16. In light of its prevalent localization, the emphasis is laid on the neural aspects.
Keywords: development; mammalian; myosin; myosin XVI; neural; neurodegenerative; unconventional.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

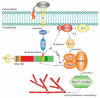

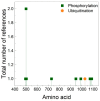
References
-
- Frénal K., Foth B.J., Soldati D. Myosin Class XIV and Other Myosins in Protists. In: Coluccio L.M., editor. Myosins, A Superfamily of Molecular Motors. Springer Netherlands; Heidelberg, Germany: 2008. pp. 421–440. (Series: Proteins and Cell Regulation).
-
- Coluccio L.M., Myosins A. Superfamily of Molecular Motors. 2nd ed. Springer International Publishing; Cham, Switzerland: 2020. (Series: Advances in Experimental Medicine and Biology).
-
- Suter D.M. Functions of Myosin Motor Proteins in the Nervous System. In: Gallo G., Lanier L., editors. Neurobiology of Actin, Advances in Neurobiology 5. Springer Science and Business Media; Berlin, Germany: 2010. pp. 45–72.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

