From Ivacaftor to Triple Combination: A Systematic Review of Efficacy and Safety of CFTR Modulators in People with Cystic Fibrosis
- PMID: 32824306
- PMCID: PMC7461566
- DOI: 10.3390/ijms21165882
From Ivacaftor to Triple Combination: A Systematic Review of Efficacy and Safety of CFTR Modulators in People with Cystic Fibrosis
Abstract
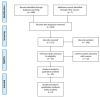
Over the last years CFTR (cystic fibrosis transmembrane conductance regulator) modulators have shown the ability to improve relevant clinical outcomes in patients with cystic fibrosis (CF). This review aims at a systematic research of the current evidence on efficacy and tolerability of CFTR modulators for different genetic subsets of patients with CF. Two investigators independently performed the search on PubMed and included phase 2 and 3 clinical trials published in the study period 1 January 2005-31 January 2020. A final pool of 23 papers was included in the systematic review for a total of 4219 patients. For each paper data of interest were extracted and reported in table. In terms of lung function, patients who had the most beneficial effects from CFTR modulation were those patients with one gating mutation receiving IVA (ivacaftor) and patients with p.Phe508del mutation, both homozygous and heterozygous, receiving ELX/TEZ/IVA (elexacaftor/tezacaftor/ivacaftor) had the most relevant beneficial effects in term of lung function, pulmonary exacerbation decrease, and symptom improvement. CFTR modulators showed an overall favorable safety profile. Next steps should aim to systematize our comprehension of scientific data of efficacy and safety coming from real life observational studies.
Keywords: CFTR modulators; clinical efficacy; cystic fibrosis; safety.
Conflict of interest statement
A.G. reports grant support and consultancy fees from Vertex Pharmaceuticals; fees from Grifols, Insmed and Chiesi Pharmaceuticals. S.A. reports grant funding or fees for consultancy from Astrazeneca, Boehringer-Ingelheim, Gilead, Glaxosmithkline, Grifols, Insmed and Zambon. R.C. reports consultancy fees from Vertex Pharmaceuticals, Gilead and Chiesi Pharmaceuticals. F.B. reports grants and consultancy fees from Astrazeneca, Bayer, Chiesi Pharmaceuticals, Insmed, Pfizer; consultancy fees from GSK, Guidotti and Grifols, Mundipharma, Vertex Pharmaceuticals, Zambon. C.C. reports consultancy fees from Vertex Pharmaceuticals, Gilead, Mylan Pharmaceutical Industries, Actelion Pharmaceuticals, and Chiesi Pharmaceuticals. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele.N Engl J Med. 2019 Nov 7;381(19):1809-1819. doi: 10.1056/NEJMoa1908639. Epub 2019 Oct 31. N Engl J Med. 2019. PMID: 31697873 Free PMC article. Clinical Trial.
-
Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial.Lancet. 2019 Nov 23;394(10212):1940-1948. doi: 10.1016/S0140-6736(19)32597-8. Epub 2019 Oct 31. Lancet. 2019. PMID: 31679946 Free PMC article. Clinical Trial.
-
VX-659-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles.N Engl J Med. 2018 Oct 25;379(17):1599-1611. doi: 10.1056/NEJMoa1807119. Epub 2018 Oct 18. N Engl J Med. 2018. PMID: 30334693 Free PMC article. Clinical Trial.
-
Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del).Cochrane Database Syst Rev. 2020 Dec 17;12(12):CD010966. doi: 10.1002/14651858.CD010966.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Nov 20;11:CD010966. doi: 10.1002/14651858.CD010966.pub4. PMID: 33331662 Free PMC article. Updated.
-
Potentiators (specific therapies for class III and IV mutations) for cystic fibrosis.Cochrane Database Syst Rev. 2019 Jan 7;1(1):CD009841. doi: 10.1002/14651858.CD009841.pub3. Cochrane Database Syst Rev. 2019. PMID: 30616300 Free PMC article.
Cited by
-
Understanding Cystic Fibrosis Comorbidities and Their Impact on Nutritional Management.Nutrients. 2022 Feb 28;14(5):1028. doi: 10.3390/nu14051028. Nutrients. 2022. PMID: 35268004 Free PMC article. Review.
-
A Review of Exocrine Pancreatic Insufficiency in Children beyond Cystic Fibrosis and the Role of Endoscopic Direct Pancreatic Function Testing.Curr Gastroenterol Rep. 2025 Feb 19;27(1):14. doi: 10.1007/s11894-025-00959-7. Curr Gastroenterol Rep. 2025. PMID: 39971805 Review.
-
Efficacy and Safety of Triple Combination Cystic Fibrosis Transmembrane Conductance Regulator Modulators in Patients With Cystic Fibrosis: A Meta-Analysis of Randomized Controlled Trials.Front Pharmacol. 2022 Mar 14;13:863280. doi: 10.3389/fphar.2022.863280. eCollection 2022. Front Pharmacol. 2022. PMID: 35359862 Free PMC article.
-
Ivacaftor-tezacaftor-elexacaftor, tezacaftor-ivacaftor and lumacaftor-ivacaftor for treating cystic fibrosis: a systematic review and economic evaluation.Health Technol Assess. 2025 May;29(19):1-111. doi: 10.3310/CPLD8546. Health Technol Assess. 2025. PMID: 40418577 Free PMC article.
-
Partial Rescue of F508del-CFTR Stability and Trafficking Defects by Double Corrector Treatment.Int J Mol Sci. 2021 May 17;22(10):5262. doi: 10.3390/ijms22105262. Int J Mol Sci. 2021. PMID: 34067708 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical