Genistein Induces Adipogenic and Autophagic Effects in Rainbow Trout (Oncorhynchus mykiss) Adipose Tissue: In Vitro and In Vivo Models
- PMID: 32824312
- PMCID: PMC7461592
- DOI: 10.3390/ijms21165884
Genistein Induces Adipogenic and Autophagic Effects in Rainbow Trout (Oncorhynchus mykiss) Adipose Tissue: In Vitro and In Vivo Models
Abstract
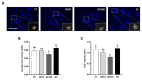
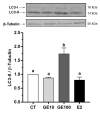
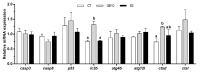
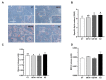
Soybeans are one of the most used alternative dietary ingredients in aquafeeds. However, they contain phytoestrogens like genistein (GE), which can have an impact on fish metabolism and health. This study aimed to investigate the in vitro and in vivo effects of GE on lipid metabolism, apoptosis, and autophagy in rainbow trout (Oncorhynchus mykiss). Primary cultured preadipocytes were incubated with GE at different concentrations, 10 or 100 μM, and 1 μM 17β-estradiol (E2). Furthermore, juveniles received an intraperitoneal injection of GE at 5 or 50 µg/g body weight, or E2 at 5 µg/g. In vitro, GE 100 μM increased lipid accumulation and reduced cell viability, apparently involving an autophagic process, indicated by the higher LC3-II protein levels, and higher lc3b and cathepsin d transcript levels achieved after GE 10 μM. In vivo, GE 50 µg/g upregulated the gene expression of fatty acid synthase (fas) and glyceraldehyde-3-phosphate dehydrogenase in adipose tissue, suggesting enhanced lipogenesis, whereas it increased hormone-sensitive lipase in liver, indicating a lipolytic response. Besides, autophagy-related genes increased in the tissues analyzed mainly after GE 50 µg/g treatment. Overall, these findings suggest that an elevated GE administration could lead to impaired adipocyte viability and lipid metabolism dysregulation in rainbow trout.
Keywords: 17β-estradiol; apoptosis; autophagy; genistein; lipid metabolism; phytoestrogens.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Screening the effects of phytoestrogens on lipid metabolism in primary cultured adipocytes from rainbow trout (Oncorhynchus mykiss) and gilthead sea bream (Sparus aurata).Fish Physiol Biochem. 2025 Mar 25;51(2):71. doi: 10.1007/s10695-025-01483-1. Fish Physiol Biochem. 2025. PMID: 40131537 Free PMC article.
-
In vitro and in vivo effects of phytoestrogens on protein turnover in rainbow trout (Oncorhynchus mykiss) white muscle.Comp Biochem Physiol C Toxicol Pharmacol. 2014 Sep;165:9-16. doi: 10.1016/j.cbpc.2014.05.003. Epub 2014 May 27. Comp Biochem Physiol C Toxicol Pharmacol. 2014. PMID: 24874080
-
Effects of phytoestrogens on growth-related and lipogenic genes in rainbow trout (Oncorhynchus mykiss).Comp Biochem Physiol C Toxicol Pharmacol. 2015 Apr;170:28-37. doi: 10.1016/j.cbpc.2015.02.001. Epub 2015 Feb 8. Comp Biochem Physiol C Toxicol Pharmacol. 2015. PMID: 25668741
-
Effects of estrogens and the phytoestrogen genistein on adipogenesis and lipogenesis in males and females.Birth Defects Res A Clin Mol Teratol. 2005 Jul;73(7):472-3. doi: 10.1002/bdra.20142. Birth Defects Res A Clin Mol Teratol. 2005. PMID: 15959885 Review. No abstract available.
-
Phytochemicals and regulation of the adipocyte life cycle.J Nutr Biochem. 2008 Nov;19(11):717-26. doi: 10.1016/j.jnutbio.2007.12.007. Epub 2008 May 20. J Nutr Biochem. 2008. PMID: 18495457 Review.
Cited by
-
The obesogenic side of Genistein.Front Endocrinol (Lausanne). 2023 Nov 30;14:1308341. doi: 10.3389/fendo.2023.1308341. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38098865 Free PMC article. Review.
-
Effects of Genistein on Lipid Metabolism, Antioxidant Activity, and Immunity of Common Carp (Cyprinus carpio L.) Fed with High-Carbohydrate and High-Fat Diets.Aquac Nutr. 2023 Mar 31;2023:9555855. doi: 10.1155/2023/9555855. eCollection 2023. Aquac Nutr. 2023. PMID: 37034827 Free PMC article.
-
Screening the effects of phytoestrogens on lipid metabolism in primary cultured adipocytes from rainbow trout (Oncorhynchus mykiss) and gilthead sea bream (Sparus aurata).Fish Physiol Biochem. 2025 Mar 25;51(2):71. doi: 10.1007/s10695-025-01483-1. Fish Physiol Biochem. 2025. PMID: 40131537 Free PMC article.
-
Impact of Hydroxytyrosol-Rich Extract Supplementation in a High-Fat Diet on Gilthead Sea Bream (Sparus aurata) Lipid Metabolism.Antioxidants (Basel). 2024 Mar 27;13(4):403. doi: 10.3390/antiox13040403. Antioxidants (Basel). 2024. PMID: 38671851 Free PMC article.
References
-
- Food and Agriculture Organization of the United Nations . The State of World Fisheries and Aquaculture. General Fisheries Commission for the Mediterranean; Rome, Italy: 2020.
-
- Turchini G.M., Torstensen B.E., Ng W.-K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2009;1:10–57. doi: 10.1111/j.1753-5131.2008.01001.x. - DOI
-
- Betancor M.B., Sprague M., Montero D., Usher S., Sayanova O., Campbell P.J., Napier J.A., Caballero M.J., Izquierdo M., Tocher D.R. Replacement of marine fish oil with de novo omega-3 oils from transgenic camelina sativa in feeds for gilthead sea bream (Sparus aurata L.) Lipids. 2016;51:1171–1191. doi: 10.1007/s11745-016-4191-4. - DOI - PMC - PubMed
-
- Calduch-Giner J.A., Sitjà-Bobadilla A., Davey G.C., Cairns M.T., Kaushik S., Pérez-Sánchez J. Dietary vegetable oils do not alter the intestine transcriptome of gilthead sea bream (Sparus aurata), but modulate the transcriptomic response to infection with Enteromyxum leei. BMC Genom. 2012;13:1–13. doi: 10.1186/1471-2164-13-470. - DOI - PMC - PubMed
-
- Pérez J.A., Rodríguez C., Bolaños A., Cejas J.R., Lorenzo A. Beef tallow as an alternative to fish oil in diets for gilthead sea bream (Sparus aurata) juveniles: Effects on fish performance, tissue fatty acid composition, health and flesh nutritional value. Eur. J. Lipid Sci. Technol. 2014;116:571–583. doi: 10.1002/ejlt.201300457. - DOI
MeSH terms
Substances
Grants and funding
- AGL2014-57974-R/Ministerio de Ciencia, Innovación y Universidades (MICIUN)
- AGL2017-89436-R/Ministerio de Ciencia, Innovación y Universidades (MICIUN)
- 2017SGR-1574/Generalitat de Catalunya
- BES-2012-061867, PRE-2018-085580, BES-2015-074654 and BES-2013-062949/Ministerio de Ciencia, Innovación y Universidades (MICIUN)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

