Applications of Photoinduced Phenomena in Supramolecularly Arranged Phthalocyanine Derivatives: A Perspective
- PMID: 32824375
- PMCID: PMC7463501
- DOI: 10.3390/molecules25163742
Applications of Photoinduced Phenomena in Supramolecularly Arranged Phthalocyanine Derivatives: A Perspective
Abstract
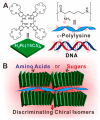
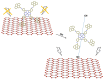
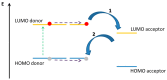
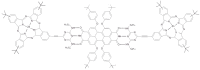
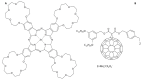
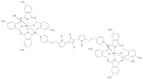
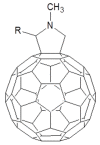
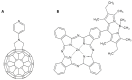
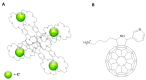
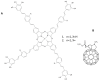
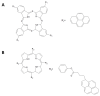
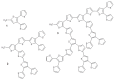
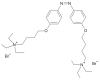
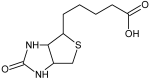
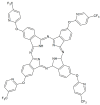
This review focuses on the description of several examples of supramolecular assemblies of phthalocyanine derivatives differently functionalized and interfaced with diverse kinds of chemical species for photo-induced phenomena applications. In fact, the role of different substituents was investigated in order to tune peculiar aggregates formation as well as, with the same aim, the possibility to interface these derivatives with other molecular species, as electron donor and acceptor, carbon allotropes, cyclodextrins, protein cages, drugs. Phthalocyanine photo-physical features are indeed really interesting and appealing but need to be preserved and optimized. Here, we highlight that the supramolecular approach is a versatile method to build up very complex and functional architectures. Further, the possibility to minimize the organization energy and to facilitate the spontaneous assembly of the molecules, in numerous examples, has been demonstrated to be more useful and performing than the covalent approach.
Keywords: Charge-transfer; PDI; UV-Vis; carbon allotropes; cyclodextrins; energy-transfer; fluorescence; fullerene; hydrogen bonds; micelles; photocatalysis; photodynamic therapy; sensors; solar cells; supramolecular assembly; time-resolved spectroscopy; π-π interaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

