Phase II Clinical Trial of Pembrolizumab in Patients with Progressive Metastatic Pheochromocytomas and Paragangliomas
- PMID: 32824391
- PMCID: PMC7465458
- DOI: 10.3390/cancers12082307
Phase II Clinical Trial of Pembrolizumab in Patients with Progressive Metastatic Pheochromocytomas and Paragangliomas
Abstract
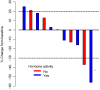
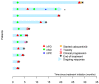
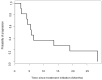
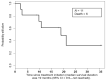
Metastatic pheochromocytomas and paragangliomas (MPPGs) are rare endocrine malignancies that are associated with high rates of morbidity and mortality because of their large tumor burden and location, progression, and release of catecholamines. Systemic therapies for MPPGs are limited. MPPGs are characterized by pseudohypoxia that may prevent immune system recognition. We conducted a phase II clinical trial of pembrolizumab in patients with progressive MPPGs. The primary endpoint was the non-progression rate at 27 weeks. The secondary endpoints included the objective response and clinical benefit rates, progression free and overall survival duration, and safety. We also determined whether PDL-1 expression and the presence of infiltrating mononuclear inflammatory cells in the primary tumor were associated with clinical response and hereditary background. Eleven patients were included in this trial, four (36%) with germline mutations and seven (64%) with hormonally active tumors. Four patients (40%, 95% confidence interval (CI) 12-74%) achieved the primary endpoint. The objective response rate was 9% (95% CI: 0-41%). The clinical benefit rate was 73% (95% CI: 39-94%). Four patients had grade 3 adverse events related to pembrolizumab. No patients experienced grade 4 or 5 adverse events or a catecholamine crisis. Progression free survival time was 5.7 months (95% CI: 4.37-not reached). The median survival duration was 19 months (95% CI: 9.9-not reached). PDL-1 expression and the presence of infiltrating mononuclear inflammatory cells in the primary tumor did not seem to be associated with disease response. Single-agent pembrolizumab has modest treatment efficacy in patients with progressive MPPGs. Positive responses seemed to be independent of patients' hereditary backgrounds, tumor hormonal status, and the presence of infiltrating mononuclear inflammatory cells or PDL-1 expression in the primary tumor.
Keywords: PD-1 inhibition; metastatic paraganglioma; metastatic pheochromocytoma; pembrolizumab; pseudohypoxia.
Conflict of interest statement
Vivek Subbiah Conflicts of interest: Research funding/Grant support for clinical trials: Roche/Genentech, Novartis, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, Pharmamar, D3, Pfizer, Multivir, Amgen, Abbvie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint medicines, Loxo oncology, Medimmune, Altum, Dragonfly therapeutics, Takeda and, National Comprehensive Cancer Network, NCI-CTEP and UT MD Anderson Cancer Center, Turning point therapeutics, Boston Pharmaceuticals. Travel: Novartis, Pharmamar, ASCO, ESMO, Helsinn, Incyte, Consultancy/Advisory board: Helsinn, LOXO Oncology/Eli Lilly, R-Pharma US, INCYTE, QED pharma, Medimmune, Novartis. Other: Medscape. Dr. Rodon Ahnert Conflicts of Interest: personal fees and other from Novartis, personal fees from Eli Lilly, personal fees from Orion Pharmaceuticals, personal fees from Peptomyc, personal fees and other from Kelun Pharmaceuticals/Klus Pharma, personal fees and other from Spectrum Pharmaceuticals Inc., personal fees and other from Pfizer, personal fees from Roche Pharmceuticals, personal fees from Ellipses Pharma, personal fees from Certera, personal fees and other from Bayer, personal fees from Ionctura SA, other from European Journal of Cancer, other from VHIO/Ministero De Empleo Y Seguridad Social, other from Chinese University of Hong Kong, other from SOLTI, other from Elsevier, other from GLAXOSMITHKLINE, other from ESMO, from Department of Defense, other from Merck Sharp & Dohme, other from Lousiania State University, other from Huntsman Cancer Institute, other from Cancer Core Europe, other from Karolinska Cancer Institute, other from King Abdullah International Medical Research Center, other from WIN Consortium, other from Janssen, other from Tocagen, other from Symphogen, other from BioAlta, other from GenMab, other from CytomX, other from Kelun-Biotech, other from Takea-Millenium, other from Ipsen, outside the submitted work; Apostolia Tsimberidou Conflicts of Interest: Research Funding (Institution): Immatics, Parker Institute for Cancer Immunotherapy, Tempus, OBI Pharma, EMD Serono, Baxalta, ONYX, Bayer, Boston Biomedical, Placon Therapeutics, Karus Therapeutics, and Tvardi Therapeutics. Consulting or Advisory Role: Covance, Genentech and Tempus. Siqing Fu Conflicts of Interest: Clinical Trial Research Support (paid to the institution). AstraZeneca; Abbisko; Anaeropharma Science; Arrien Pharmaceuticals; BeiGene; BioAtla, LLC; Boehringer Ingelheim; Eli Lilly & Co.; Hookipa Biotech GmBH; Huya Bioscience International; IMV, Inc.; Innovent Biologics, Co., Ltd.; Lyvgen Biopharm, Co., Ltd.; MacroGenics; Medivir AB; Millennium Pharmaceuticals, Inc.; Nerviano Medical Sciences; NeuPharma, Inc.; NIH/NCI; Novartis; OncoMed Pharmaceuticals; Parexel International, LLC; Sellas Life Sciences Group; Soricimed Biopharma, Inc.; Tolero Pharmaceuticals. Aung Naing Conflict of interest: Research funding from NCI; EMD Serono; MedImmune; HealiosOnc. Nutrition; Atterocor; Amplimmune; ARMO BioSciences; Eli Lilly; KaryopharmTherapeutics; Incyte; Novartis; Regeneron; Merck; BMS; Pfizer, CytomXTherapeutics; Neon Therapeutics; CalitheraBiosciences; TopAllianceBiosciences; Kymab; PsiOxus; Immune Deficiency Foundation (Spouse). On advisory board of CytomXTherapeutics, Novartis, Kymab, Genome. Travel and accommodation expense from ARMO BioSciences. The following Authors do not have Conflicts of Interest: Camilo Jimenez, Bettzy Stephen, Junsheng Ma, Denai Milton, Mingxuan Xu, Abdualrazzak Zarifa, Fechukwu Omolara Akhmedzhanov, Mouhammed Habra.
Figures
References
-
- Butz J.J., Yan Q., McKenzie T.J., Weingarten T.N., Cavalcante A.N., Bancos I., Young W.F., Jr., Schroeder D.R., Martin D.P., Sprung J. Perioperative outcomes of syndromic paraganglioma and pheochromocytoma resection in patients with von Hippel-Lindau disease, multiple endocrine neoplasia type 2, or neurofibromatosis type 1. Surgery. 2017;162:1259–1269. doi: 10.1016/j.surg.2017.08.002. - DOI - PubMed
-
- Ayala-Ramirez M., Feng L., Johnson M.M., Ejaz S., Habra M.A., Rich T., Busaidy N., Cote G.J., Perrier N., Phan A., et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: Primary tumor size and primary tumor location as prognostic indicators. J. Clin. Endocrinol. Metab. 2011;96:717–725. doi: 10.1210/jc.2010-1946. - DOI - PubMed
-
- Ayala-Ramirez M., Palmer J.L., Hofmann M.C., de la Cruz M., Moon B.S., Waguespack S.G., Habra M.A., Jimenez C. Bone metastases and skeletal-related events in patients with malignant pheochromocytoma and sympathetic paraganglioma. J. Clin. Endocrinol. Metab. 2013;98:1492–1497. doi: 10.1210/jc.2012-4231. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

