Liquid-Liquid Phase Separation in Crowded Environments
- PMID: 32824618
- PMCID: PMC7460619
- DOI: 10.3390/ijms21165908
Liquid-Liquid Phase Separation in Crowded Environments
Abstract
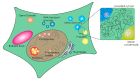
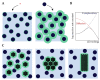
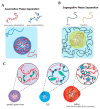
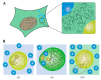
Biomolecular condensates play a key role in organizing cellular fluids such as the cytoplasm and nucleoplasm. Most of these non-membranous organelles show liquid-like properties both in cells and when studied in vitro through liquid-liquid phase separation (LLPS) of purified proteins. In general, LLPS of proteins is known to be sensitive to variations in pH, temperature and ionic strength, but the role of crowding remains underappreciated. Several decades of research have shown that macromolecular crowding can have profound effects on protein interactions, folding and aggregation, and it must, by extension, also impact LLPS. However, the precise role of crowding in LLPS is far from trivial, as most condensate components have a disordered nature and exhibit multiple weak attractive interactions. Here, we discuss which factors determine the scope of LLPS in crowded environments, and we review the evidence for the impact of macromolecular crowding on phase boundaries, partitioning behavior and condensate properties. Based on a comparison of both in vivo and in vitro LLPS studies, we propose that phase separation in cells does not solely rely on attractive interactions, but shows important similarities to segregative phase separation.
Keywords: crowding; intrinsically disordered proteins; liquid–liquid phase separation; membraneless organelles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

