Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update
- PMID: 32824664
- PMCID: PMC7460570
- DOI: 10.3390/ijms21165920
Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update
Abstract
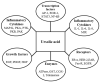
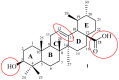
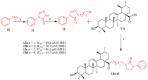
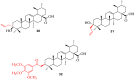
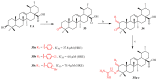
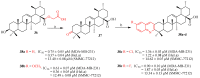
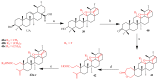
Ursolic acid is a pharmacologically active pentacyclic triterpenoid derived from medicinal plants, fruit, and vegetables. The pharmacological activities of ursolic acid have been extensively studied over the past few years and various reports have revealed that ursolic acid has multiple biological activities, which include anti-inflammatory, antioxidant, anti-cancer, etc. In terms of cancer treatment, ursolic acid interacts with a number of molecular targets that play an essential role in many cell signaling pathways. It suppresses transformation, inhibits proliferation, and induces apoptosis of tumor cells. Although ursolic acid has many benefits, its therapeutic applications in clinical medicine are limited by its poor bioavailability and absorption. To overcome such disadvantages, researchers around the globe have designed and developed synthetic ursolic acid derivatives with enhanced therapeutic effects by structurally modifying the parent skeleton of ursolic acid. These structurally modified compounds display enhanced therapeutic effects when compared to ursolic acid. This present review summarizes various synthesized derivatives of ursolic acid with anti-cancer activity which were reported from 2015 to date.
Keywords: anti-cancer activity; derivatives/analogs; structural modification; ursolic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

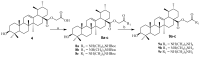
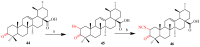
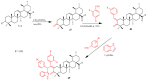
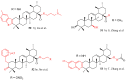
References
-
- Sisodiya P.S. Plant Derived Anticancer Agents: A Review. Int. J. Res. Dev. Pharm. Life Sci. 2013;2:293–308.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

