TXNIP/TBP-2: A Master Regulator for Glucose Homeostasis
- PMID: 32824669
- PMCID: PMC7464905
- DOI: 10.3390/antiox9080765
TXNIP/TBP-2: A Master Regulator for Glucose Homeostasis
Abstract
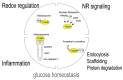
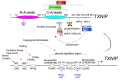
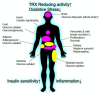
Identification of thioredoxin binding protein-2 (TBP-2), which is currently known as thioredoxin interacting protein (TXNIP), as an important binding partner for thioredoxin (TRX) revealed that an evolutionarily conserved reduction-oxidation (redox) signal complex plays an important role for pathophysiology. Due to the reducing activity of TRX, the TRX/TXNIP signal complex has been shown to be an important regulator for redox-related signal transduction in many types of cells in various species. In addition to its role in redox-dependent regulation, TXNIP has cellular functions that are performed in a redox-independent manner, which largely rely on their scaffolding function as an ancestral α-Arrestin family. Both the redox-dependent and -independent TXNIP functions serve as regulatory pathways in glucose metabolism. This review highlights the key advances in understanding TXNIP function as a master regulator for whole-body glucose homeostasis. The potential for therapeutic advantages of targeting TXNIP in diabetes and the future direction of the study are also discussed.
Keywords: TBP-2; TXNIP; glucose homeostasis.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures
Similar articles
-
Thioredoxin interacting protein: redox dependent and independent regulatory mechanisms.Antioxid Redox Signal. 2012 Mar 15;16(6):587-96. doi: 10.1089/ars.2011.4137. Epub 2011 Dec 20. Antioxid Redox Signal. 2012. PMID: 21929372 Free PMC article. Review.
-
Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases.Front Immunol. 2014 Jan 9;4:514. doi: 10.3389/fimmu.2013.00514. Front Immunol. 2014. PMID: 24409188 Free PMC article. Review.
-
Physiological and Pathophysiological Roles of Thioredoxin Interacting Protein: A Perspective on Redox Inflammation and Metabolism.Antioxid Redox Signal. 2023 Feb;38(4-6):442-460. doi: 10.1089/ars.2022.0022. Antioxid Redox Signal. 2023. PMID: 35754346 Free PMC article. Review.
-
The structural basis for the negative regulation of thioredoxin by thioredoxin-interacting protein.Nat Commun. 2014;5:2958. doi: 10.1038/ncomms3958. Nat Commun. 2014. PMID: 24389582 Free PMC article.
-
Thioredoxin-Interacting Protein in Cancer and Diabetes.Antioxid Redox Signal. 2022 May;36(13-15):1001-1022. doi: 10.1089/ars.2021.0038. Epub 2021 Oct 7. Antioxid Redox Signal. 2022. PMID: 34384271 Free PMC article. Review.
Cited by
-
Dual regulation of TxNIP by ChREBP and FoxO1 in liver.iScience. 2021 Feb 20;24(3):102218. doi: 10.1016/j.isci.2021.102218. eCollection 2021 Mar 19. iScience. 2021. PMID: 33748706 Free PMC article.
-
LMNA R482L mutation causes impairments in C2C12 myoblasts subpopulations, alterations in metabolic reprogramming during differentiation, and oxidative stress.Sci Rep. 2025 Feb 13;15(1):5358. doi: 10.1038/s41598-025-88219-6. Sci Rep. 2025. PMID: 39948343 Free PMC article.
-
The Emerging Role of Metabolism in Brain-Heart Axis: New Challenge for the Therapy and Prevention of Alzheimer Disease. May Thioredoxin Interacting Protein (TXNIP) Play a Role?Biomolecules. 2021 Nov 8;11(11):1652. doi: 10.3390/biom11111652. Biomolecules. 2021. PMID: 34827650 Free PMC article. Review.
-
The Importance of Thioredoxin-1 in Health and Disease.Antioxidants (Basel). 2023 May 11;12(5):1078. doi: 10.3390/antiox12051078. Antioxidants (Basel). 2023. PMID: 37237944 Free PMC article. Review.
-
Effects of Quercitrin on PRV-Induced Secretion of Reactive Oxygen Species and Prediction of lncRNA Regulatory Targets in 3D4/2 Cells.Antioxidants (Basel). 2022 Mar 25;11(4):631. doi: 10.3390/antiox11040631. Antioxidants (Basel). 2022. PMID: 35453316 Free PMC article.
References
-
- Nishiyama A., Matsui M., Iwata S., Hirota K., Masutani H., Nakamura H., Takagi Y., Sono H., Gon Y., Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

