Necroptosis in Hepatosteatotic Ischaemia-Reperfusion Injury
- PMID: 32824744
- PMCID: PMC7460692
- DOI: 10.3390/ijms21165931
Necroptosis in Hepatosteatotic Ischaemia-Reperfusion Injury
Abstract
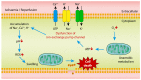
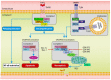
While liver transplantation remains the sole treatment option for patients with end-stage liver disease, there are numerous limitations to liver transplantation including the scarcity of donor livers and a rise in livers that are unsuitable to transplant such as those with excess steatosis. Fatty livers are susceptible to ischaemia-reperfusion (IR) injury during transplantation and IR injury results in primary graft non-function, graft failure and mortality. Recent studies have described new cell death pathways which differ from the traditional apoptotic pathway. Necroptosis, a regulated form of cell death, has been associated with hepatic IR injury. Receptor-interacting protein kinase 3 (RIPK3) and mixed-lineage kinase domain-like pseudokinase (MLKL) are thought to be instrumental in the execution of necroptosis. The study of hepatic necroptosis and potential therapeutic approaches to attenuate IR injury will be a key factor in improving our knowledge regarding liver transplantation with fatty donor livers. In this review, we focus on the effect of hepatic steatosis during liver transplantation as well as molecular mechanisms of necroptosis and its involvement during liver IR injury. We also discuss the immune responses triggered during necroptosis and examine the utility of necroptosis inhibitors as potential therapeutic approaches to alleviate IR injury.
Keywords: ischaemia-reperfusion injury; liver transplantation; necroptosis; non-alcoholic fatty liver disease; steatosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Luckett K., Kaiser T.E., Bari K., Safdar K., Schoech M.R., Apewokin S., Diwan T.S., Cuffy M.C., Anwar N., Shah S.A. Use of Hepatitis C Virus Antibody-Positive Donor Livers in Hepatitis C Nonviremic Liver Transplant Recipients. J. Am. Coll. Surg. 2019;228:560–567. doi: 10.1016/j.jamcollsurg.2018.12.004. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

