Electrochemical Detection of Neurotransmitters
- PMID: 32824869
- PMCID: PMC7459656
- DOI: 10.3390/bios10080101
Electrochemical Detection of Neurotransmitters
Abstract
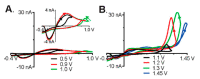
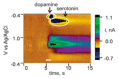
Neurotransmitters are important chemical messengers in the nervous system that play a crucial role in physiological and physical health. Abnormal levels of neurotransmitters have been correlated with physical, psychotic, and neurodegenerative diseases such as Alzheimer's, Parkinson's, dementia, addiction, depression, and schizophrenia. Although multiple neurotechnological approaches have been reported in the literature, the detection and monitoring of neurotransmitters in the brain remains a challenge and continues to garner significant attention. Neurotechnology that provides high-throughput, as well as fast and specific quantification of target analytes in the brain, without negatively impacting the implanted region is highly desired for the monitoring of the complex intercommunication of neurotransmitters. Therefore, it is crucial to develop clinical assessment techniques that are sensitive and reliable to monitor and modulate these chemical messengers and screen diseases. This review focuses on summarizing the current electrochemical measurement techniques that are capable of sensing neurotransmitters with high temporal resolution in real time. Advanced neurotransmitter sensing platforms that integrate nanomaterials and biorecognition elements are explored.
Keywords: biosensors; cyclic voltammetry; differential pulse voltammetry; electrochemical; fast scan cyclic voltammetry; neurotransmitters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
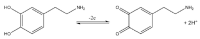
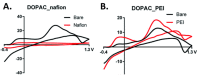
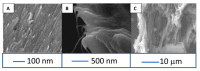

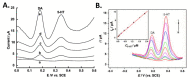
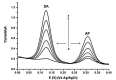
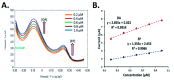
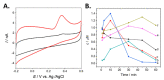
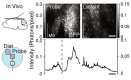

References
-
- Chauhan N., Soni S., Agrawal P., Balhara Y.P.S., Jain U. Recent advancement in nanosensors for neurotransmitters detection: Present and future perspective. Process. Biochem. 2020;91:241–259. doi: 10.1016/j.procbio.2019.12.016. - DOI
-
- Slaughter G., Robinson M., Tyson J., Zhang C.J. Neuroelectronic device process development and challenge. Opt. Microlithogr. XXX. 2017;10147:101470W. doi: 10.1117/12.2256297. - DOI
-
- Aoki I., Shirane K., Tokimoto T., Nakagawa K. Separation of fine particles using rotating tube with alternate flow. Rev. Sci. Instrum. 1986;57:2859–2861. doi: 10.1063/1.1139056. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

