Annexin A2 Mediates Dysferlin Accumulation and Muscle Cell Membrane Repair
- PMID: 32824910
- PMCID: PMC7565960
- DOI: 10.3390/cells9091919
Annexin A2 Mediates Dysferlin Accumulation and Muscle Cell Membrane Repair
Abstract
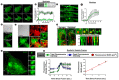
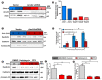
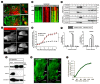
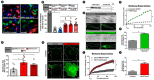
Muscle cell plasma membrane is frequently damaged by mechanical activity, and its repair requires the membrane protein dysferlin. We previously identified that, similar to dysferlin deficit, lack of annexin A2 (AnxA2) also impairs repair of skeletal myofibers. Here, we have studied the mechanism of AnxA2-mediated muscle cell membrane repair in cultured muscle cells. We find that injury-triggered increase in cytosolic calcium causes AnxA2 to bind dysferlin and accumulate on dysferlin-containing vesicles as well as with dysferlin at the site of membrane injury. AnxA2 accumulates on the injured plasma membrane in cholesterol-rich lipid microdomains and requires Src kinase activity and the presence of cholesterol. Lack of AnxA2 and its failure to translocate to the plasma membrane, both prevent calcium-triggered dysferlin translocation to the plasma membrane and compromise repair of the injured plasma membrane. Our studies identify that Anx2 senses calcium increase and injury-triggered change in plasma membrane cholesterol to facilitate dysferlin delivery and repair of the injured plasma membrane.
Keywords: muscle injury; muscular dystrophy; plasma membrane; vesicle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Annexin A2 links poor myofiber repair with inflammation and adipogenic replacement of the injured muscle.Hum Mol Genet. 2017 Jun 1;26(11):1979-1991. doi: 10.1093/hmg/ddx065. Hum Mol Genet. 2017. PMID: 28334824 Free PMC article.
-
Fibroadipogenic progenitors are responsible for muscle loss in limb girdle muscular dystrophy 2B.Nat Commun. 2019 Jun 3;10(1):2430. doi: 10.1038/s41467-019-10438-z. Nat Commun. 2019. PMID: 31160583 Free PMC article.
-
Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing.J Biol Chem. 2003 Dec 12;278(50):50466-73. doi: 10.1074/jbc.M307247200. Epub 2003 Sep 23. J Biol Chem. 2003. PMID: 14506282
-
Muscle Cells Fix Breaches by Orchestrating a Membrane Repair Ballet.J Neuromuscul Dis. 2018;5(1):21-28. doi: 10.3233/JND-170251. J Neuromuscul Dis. 2018. PMID: 29480214 Free PMC article. Review.
-
Dysferlin function in skeletal muscle: Possible pathological mechanisms and therapeutical targets in dysferlinopathies.Exp Neurol. 2016 Sep;283(Pt A):246-54. doi: 10.1016/j.expneurol.2016.06.026. Epub 2016 Jun 25. Exp Neurol. 2016. PMID: 27349407 Review.
Cited by
-
Oxidative Stress, Inflammation and Connexin Hemichannels in Muscular Dystrophies.Biomedicines. 2022 Feb 21;10(2):507. doi: 10.3390/biomedicines10020507. Biomedicines. 2022. PMID: 35203715 Free PMC article. Review.
-
Role of calcium-sensor proteins in cell membrane repair.Biosci Rep. 2023 Feb 27;43(2):BSR20220765. doi: 10.1042/BSR20220765. Biosci Rep. 2023. PMID: 36728029 Free PMC article. Review.
-
Anoctamin 5 Knockout Mouse Model Recapitulates LGMD2L Muscle Pathology and Offers Insight Into in vivo Functional Deficits.J Neuromuscul Dis. 2021;8(s2):S243-S255. doi: 10.3233/JND-210720. J Neuromuscul Dis. 2021. PMID: 34633328 Free PMC article.
-
Annexin A2 in Fibrinolysis, Inflammation and Fibrosis.Int J Mol Sci. 2021 Jun 25;22(13):6836. doi: 10.3390/ijms22136836. Int J Mol Sci. 2021. PMID: 34202091 Free PMC article. Review.
-
Human skeletal muscle fiber heterogeneity beyond myosin heavy chains.Nat Commun. 2025 Feb 19;16(1):1764. doi: 10.1038/s41467-025-56896-6. Nat Commun. 2025. PMID: 39971958 Free PMC article.
References
-
- Bashir R., Britton S., Strachan T., Keers S., Vafiadaki E., Lako M., Richard I., Marchand S., Bourg N., Argov Z., et al. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat. Genet. 1998;20:37–42. doi: 10.1038/1689. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

