Immunotherapy for Glioblastoma: Current State, Challenges, and Future Perspectives
- PMID: 32824974
- PMCID: PMC7565291
- DOI: 10.3390/cancers12092334
Immunotherapy for Glioblastoma: Current State, Challenges, and Future Perspectives
Abstract
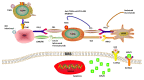
Glioblastoma is the most lethal intracranial primary malignancy by no optimal treatment option. Cancer immunotherapy has achieved remarkable survival benefits against various advanced tumors, such as melanoma and non-small-cell lung cancer, thus triggering great interest as a new therapeutic strategy for glioblastoma. Moreover, the central nervous system has been rediscovered recently as a region for active immunosurveillance. There are vibrant investigations for successful glioblastoma immunotherapy despite the fact that initial clinical trial results are somewhat disappointing with unique challenges including T-cell dysfunction in the patients. This review will explore the potential of current immunotherapy modalities for glioblastoma treatment, especially focusing on major immune checkpoint inhibitors and the future strategies with novel targets and combo therapies. Immune-related adverse events and clinical challenges in glioblastoma immunotherapy are also summarized. Glioblastoma provides persistent difficulties for immunotherapy with a complex state of patients' immune dysfunction and a variety of constraints in drug delivery to the central nervous system. However, rational design of combinational regimens and new focuses on myeloid cells and novel targets to circumvent current limitations hold promise to advent truly viable immunotherapy for glioblastoma.
Keywords: glioblastoma; immune-checkpoint inhibitors; immune-related adverse events; tumor microenvironment; tumor-associated macrophages and microglia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-Year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Ostrom Q.T., Gittleman H., Fulop J., Liu M., Blanda R., Kromer C., Wolinsky Y., Kruchko C., Barnholtz-Sloan J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the united states in 2008-2012. Neuro-Oncology. 2015;17:v1–v62. doi: 10.1093/neuonc/nov189. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

