Piceatannol, a Natural Analog of Resveratrol, Exerts Anti-angiogenic Efficiencies by Blockage of Vascular Endothelial Growth Factor Binding to Its Receptor
- PMID: 32824997
- PMCID: PMC7504081
- DOI: 10.3390/molecules25173769
Piceatannol, a Natural Analog of Resveratrol, Exerts Anti-angiogenic Efficiencies by Blockage of Vascular Endothelial Growth Factor Binding to Its Receptor
Abstract
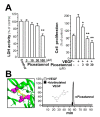
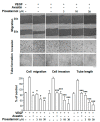
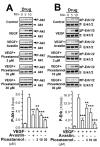
Piceatannol is also named as trans-3,4,3',5'-tetrahydroxy-stilbene, which is a natural analog of resveratrol and a polyphenol existing in red wine, grape and sugar cane. Piceatannol has been proved to possess activities of immunomodulatory, anti-inflammatory, antiproliferative and anticancer. However, the effect of piceatannol on VEGF-mediated angiogenesis is not known. Here, the inhibitory effects of piceatannol on VEGF-induced angiogenesis were tested both in vitro and in vivo models of angiogenesis. In human umbilical vein endothelial cells (HUVECs), piceatannol markedly reduced the VEGF-induced cell proliferation, migration, invasion, as well as tube formation without affecting cell viability. Furthermore, piceatannol significantly inhibited the formation of subintestinal vessel in zebrafish embryos in vivo. In addition, we identified the underlying mechanism of piceatannol in triggering the anti-angiogenic functions. Piceatannol was proposed to bind with VEGF, thus attenuating VEGF in activating VEGF receptor and blocking VEGF-mediated downstream signaling, including expressions of phosphorylated eNOS, Erk and Akt. Furthermore, piceatannol visibly suppressed ROS formation, as triggered by VEGF. Moreover, we further determined the outcome of piceatannol binding to VEGF in cancer cells: piceatannol significantly suppressed VEGF-induced colon cancer proliferation and migration. Thus, these lines of evidence supported the conclusion that piceatannol could down regulate the VEGF-mediated angiogenic functions with no cytotoxicity via decreasing the amount of VEGF binding to its receptors, thus affecting the related downstream signaling. Piceatannol may be developed into therapeutic agents or health products to reduce the high incidence of angiogenesis-related diseases.
Keywords: VEGF; VEGFR2; herbal medicine; piceatannol; pure compound; vasculogenesis.
Conflict of interest statement
All of the authors have no conflicts of interest to declare.
Figures





Similar articles
-
Binding of Resveratrol to Vascular Endothelial Growth Factor Suppresses Angiogenesis by Inhibiting the Receptor Signaling.J Agric Food Chem. 2019 Jan 30;67(4):1127-1137. doi: 10.1021/acs.jafc.8b05977. Epub 2019 Jan 17. J Agric Food Chem. 2019. PMID: 30525561
-
Polydatin suppresses VEGF-induced angiogenesis through binding with VEGF and inhibiting its receptor signaling.FASEB J. 2019 Jan;33(1):532-544. doi: 10.1096/fj.201800750R. Epub 2018 Jul 10. FASEB J. 2019. PMID: 29989844
-
Eriocalyxin B, a natural diterpenoid, inhibited VEGF-induced angiogenesis and diminished angiogenesis-dependent breast tumor growth by suppressing VEGFR-2 signaling.Oncotarget. 2016 Dec 13;7(50):82820-82835. doi: 10.18632/oncotarget.12652. Oncotarget. 2016. PMID: 27756875 Free PMC article.
-
Exploring the Intracrine Functions of VEGF-A.Biomolecules. 2021 Jan 19;11(1):128. doi: 10.3390/biom11010128. Biomolecules. 2021. PMID: 33478167 Free PMC article. Review.
-
Canonical and noncanonical vascular endothelial growth factor pathways: new developments in biology and signal transduction.Arterioscler Thromb Vasc Biol. 2015 Jan;35(1):30-9. doi: 10.1161/ATVBAHA.114.303215. Epub 2014 Oct 2. Arterioscler Thromb Vasc Biol. 2015. PMID: 25278287 Free PMC article. Review.
Cited by
-
Dual Effects of Resveratrol on the Expression and Secretion of Angiogenic Factors.Int J Mol Cell Med. 2022;11(1):16-30. doi: 10.22088/IJMCM.BUMS.11.1.16. Epub 2022 Oct 3. Int J Mol Cell Med. 2022. PMID: 36397806 Free PMC article.
-
Immunomodulatory Role of Plants and Their Constituents on the Management of Metabolic Disorders: An Evidence-Based Review.Drug Des Devel Ther. 2024 Feb 23;18:513-534. doi: 10.2147/DDDT.S442566. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38415194 Free PMC article. Review.
-
Rhus coriaria L. (Sumac) Demonstrates Oncostatic Activity in the Therapeutic and Preventive Model of Breast Carcinoma.Int J Mol Sci. 2020 Dec 26;22(1):183. doi: 10.3390/ijms22010183. Int J Mol Sci. 2020. PMID: 33375383 Free PMC article.
-
Autophagy Activation Promoted by Pulses of Light and Phytochemicals Counteracting Oxidative Stress during Age-Related Macular Degeneration.Antioxidants (Basel). 2023 May 30;12(6):1183. doi: 10.3390/antiox12061183. Antioxidants (Basel). 2023. PMID: 37371913 Free PMC article. Review.
-
Redox Homeostasis and Nrf2-Regulated Mechanisms Are Relevant to Male Infertility.Antioxidants (Basel). 2024 Feb 3;13(2):193. doi: 10.3390/antiox13020193. Antioxidants (Basel). 2024. PMID: 38397791 Free PMC article. Review.
References
-
- Folkman J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. - PubMed
-
- Bagherpoorfard M., Soheili A.R. A moving mesh method for mathematical model of capillary formation in tumor angiogenesis. Iran. J. Sci. Technol. Trans. A. Sci. 2019;43:1745–1753. doi: 10.1007/s40995-018-0623-8. - DOI
-
- Matsuoka A., Mizumoto Y., Ono M., Kagami K., Obata T., Terakawa J., Maida Y., Nakamura M., Daikoku T., Fujiwara H. Novel strategy of ovarian cancer implantation: Pre-invasive growth of fibrin-anchored cells with neovascularization. Cancer Sci. 2019;110:2658–2666. doi: 10.1111/cas.14098. - DOI - PMC - PubMed
-
- Folkman J., Shing Y. Angiogenesis. J. Biol. Chem. 1992;267:10931–10934. - PubMed
MeSH terms
Substances
Grants and funding
- ZDSYS201707281432317; JCYJ20170413173747440; JCYJ20180306174903174/Shenzhen Science and Technology Innovation Committee
- 2019M653087/China Post-doctoral Science Foundation
- ZSST20SC03/Zhongshan Municipal Bureau of Science and Technology
- GZSTI16SC02; GZSTI17SC02/Guangzhou Science and Technology Committee Research Grant
- T13-607/12R/Hong Kong RGC Theme-based Research Scheme
LinkOut - more resources
Full Text Sources
Miscellaneous

