Evaluation of the Usefulness of CO-RADS for Chest CT in Patients Suspected of Having COVID-19
- PMID: 32825060
- PMCID: PMC7555303
- DOI: 10.3390/diagnostics10090608
Evaluation of the Usefulness of CO-RADS for Chest CT in Patients Suspected of Having COVID-19
Abstract
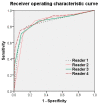
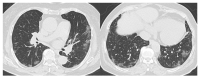
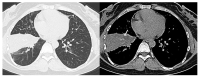
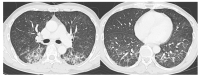
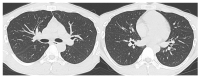
The purpose of this study was to use the Coronavirus Disease 2019 (COVID-19) Reporting and Data System (CO-RADS) to evaluate the chest computed tomography (CT) images of patients suspected of having COVID-19, and to investigate its diagnostic performance and interobserver agreement. The Dutch Radiological Society developed CO-RADS as a diagnostic indicator for assessing suspicion of lung involvement of COVID-19 on a scale of 1 (very low) to 5 (very high). We investigated retrospectively 154 adult patients with clinically suspected COVID-19, between April and June 2020, who underwent chest CT and reverse transcription-polymerase chain reaction (RT-PCR). The patients' average age was 61.3 years (range, 21-93), 101 were male, and 76 were RT-PCR positive. Using CO-RADS, four radiologists evaluated the chest CT images. Sensitivity, specificity, and area under the receiver operating characteristic curve (AUC) were calculated. Interobserver agreement was calculated using the intraclass correlation coefficient (ICC) by comparing the individual reader's score to the median of the remaining three radiologists. The average sensitivity was 87.8% (range, 80.2-93.4%), specificity was 66.4% (range, 51.3-84.5%), and AUC was 0.859 (range, 0.847-0.881); there was no significant difference between the readers (p > 0.200). In 325 (52.8%) of 616 observations, there was absolute agreement among observers. The average ICC of readers was 0.840 (range, 0.800-0.874; p < 0.001). CO-RADS is a categorical taxonomic evaluation scheme for COVID-19 pneumonia, using chest CT images, that provides outstanding performance and from substantial to almost perfect interobserver agreement for predicting COVID-19.
Keywords: CO-RADS; COVID-19; chest imaging; computed tomography; pneumonia; radiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Novel Coronavirus (2019-nCoV) Situation Reports. [(accessed on 10 August 2020)]; Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio....
-
- Riccò M., Ferraro P., Gualerzi G., Ranzieri S., Henry B.M., Said Y.B., Pyatigorskaya N.V., Nevolina E., Wu J., Bragazzi N.L., et al. Point-of-care diagnostic tests for detecting SARS-CoV-2 antibodies: A systematic review and meta-analysis of real-world data. J. Clin. Med. 2020;9:1515. doi: 10.3390/jcm9051515. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

