The Sialoside-Binding Pocket of SARS-CoV-2 Spike Glycoprotein Structurally Resembles MERS-CoV
- PMID: 32825063
- PMCID: PMC7551769
- DOI: 10.3390/v12090909
The Sialoside-Binding Pocket of SARS-CoV-2 Spike Glycoprotein Structurally Resembles MERS-CoV
Abstract
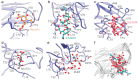
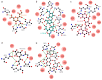
COVID-19 novel coronavirus (CoV) disease caused by severe acquired respiratory syndrome (SARS)-CoV-2 manifests severe lethal respiratory illness in humans and has recently developed into a worldwide pandemic. The lack of effective treatment strategy and vaccines against the SARS-CoV-2 poses a threat to human health. An extremely high infection rate and multi-organ secondary infection within a short period of time makes this virus more deadly and challenging for therapeutic interventions. Despite high sequence similarity and utilization of common host-cell receptor, human angiotensin-converting enzyme-2 (ACE2) for virus entry, SARS-CoV-2 is much more infectious than SARS-CoV. Structure-based sequence comparison of the N-terminal domain (NTD) of the spike protein of Middle East respiratory syndrome (MERS)-CoV, SARS-CoV, and SARS-CoV-2 illustrate three divergent loop regions in SARS-CoV-2, which is reminiscent of MERS-CoV sialoside binding pockets. Comparative binding analysis with host sialosides revealed conformational flexibility of SARS-CoV-2 divergent loop regions to accommodate diverse glycan-rich sialosides. These key differences with SARS-CoV and similarity with MERS-CoV suggest an evolutionary adaptation of SARS-CoV-2 spike glycoprotein reciprocal interaction with host surface sialosides to infect host cells with wide tissue tropism.
Keywords: MERS-CoV; N-terminal domain; SARS-CoV-2; spike glycoprotein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

