The Pathogenesis of Systemic Sclerosis: An Understanding Based on a Common Pathologic Cascade across Multiple Organs and Additional Organ-Specific Pathologies
- PMID: 32825112
- PMCID: PMC7565034
- DOI: 10.3390/jcm9092687
The Pathogenesis of Systemic Sclerosis: An Understanding Based on a Common Pathologic Cascade across Multiple Organs and Additional Organ-Specific Pathologies
Abstract
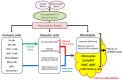
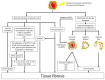
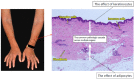
Systemic sclerosis (SSc) is a multisystem autoimmune and vascular disease resulting in fibrosis of various organs with unknown etiology. Accumulating evidence suggests that a common pathologic cascade across multiple organs and additional organ-specific pathologies underpin SSc development. The common pathologic cascade starts with vascular injury due to autoimmune attacks and unknown environmental factors. After that, dysregulated angiogenesis and defective vasculogenesis promote vascular structural abnormalities, such as capillary loss and arteriolar stenosis, while aberrantly activated endothelial cells facilitate the infiltration of circulating immune cells into perivascular areas of various organs. Arteriolar stenosis directly causes pulmonary arterial hypertension, scleroderma renal crisis and digital ulcers. Chronic inflammation persistently activates interstitial fibroblasts, leading to the irreversible fibrosis of multiple organs. The common pathologic cascade interacts with a variety of modifying factors in each organ, such as keratinocytes and adipocytes in the skin, esophageal stratified squamous epithelia and myenteric nerve system in gastrointestinal tract, vasospasm of arterioles in the heart and kidney, and microaspiration of gastric content in the lung. To better understand SSc pathogenesis and develop new disease-modifying therapies, it is quite important to understand the complex pathogenesis of SSc from the two distinct perspectives, namely the common pathologic cascade and additional organ-specific pathologies.
Keywords: a common pathologic cascade across multiple organs; additional organ-specific pathologies; systemic sclerosis.
Conflict of interest statement
The author declare no conflict of interest.
Figures

References
-
- Arnett F.C., Cho M., Chatterjee S., Aguilar M.B., Reveille J.D., Mayes M.D. Familial occurrence frequencies and relative risks for systemic sclerosis (scleroderma) in three United States cohorts. Arthritis Rheum. 2001;44:1359–1362. doi: 10.1002/1529-0131(200106)44:6<1359::AID-ART228>3.0.CO;2-S. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

