Novel On-Demand 3-Dimensional (3-D) Printed Tablets Using Fill Density as an Effective Release-Controlling Tool
- PMID: 32825229
- PMCID: PMC7564432
- DOI: 10.3390/polym12091872
Novel On-Demand 3-Dimensional (3-D) Printed Tablets Using Fill Density as an Effective Release-Controlling Tool
Abstract
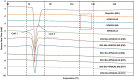
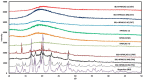
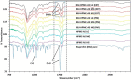
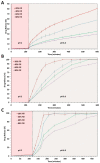
This research demonstrates the use of fill density as an effective tool for controlling the drug release without changing the formulation composition. The merger of hot-melt extrusion (HME) with fused deposition modeling (FDM)-based 3-dimensional (3-D) printing processes over the last decade has directed pharmaceutical research towards the possibility of printing personalized medication. One key aspect of printing patient-specific dosage forms is controlling the release dynamics based on the patient's needs. The purpose of this research was to understand the impact of fill density and interrelate it with the release of a poorly water-soluble, weakly acidic, active pharmaceutical ingredient (API) from a hydroxypropyl methylcellulose acetate succinate (HPMC-AS) matrix, both mathematically and experimentally. Amorphous solid dispersions (ASDs) of ibuprofen with three grades of AquaSolveTM HPMC-AS (HG, MG, and LG) were developed using an HME process and evaluated using solid-state characterization techniques. Differential scanning calorimetry (DSC), powder X-ray diffraction (pXRD), and polarized light microscopy (PLM) confirmed the amorphous state of the drug in both polymeric filaments and 3D printed tablets. The suitability of the manufactured filaments for FDM processes was investigated using texture analysis (TA) which showed robust mechanical properties of the developed filament compositions. Using FDM, tablets with different fill densities (20-80%) and identical dimensions were printed for each polymer. In vitro pH shift dissolution studies revealed that the fill density has a significant impact (F(11, 24) = 15,271.147, p < 0.0001) and a strong negative correlation (r > -0.99; p < 0.0001) with the release performance, where 20% infill demonstrated the fastest and most complete release, whereas 80% infill depicted a more controlled release. The results obtained from this research can be used to develop a robust formulation strategy to control the drug release from 3D printed dosage forms as a function of fill density.
Keywords: HPMC-AS; additive manufacturing; amorphous solid dispersions; controlled release; fused deposition modeling; hot-melt extrusion; personalized medication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Challenges, current status and emerging strategies in the development of rapidly dissolving FDM 3D-printed tablets: An overview and commentary.ADMET DMPK. 2023 Jan 1;11(1):33-55. doi: 10.5599/admet.1622. eCollection 2023. ADMET DMPK. 2023. PMID: 36778904 Free PMC article. Review.
-
Three-Dimensional Printing of Personalized Carbamazepine Tablets Using Hydrophilic Polymers: An Investigation of Correlation Between Dissolution Kinetics and Printing Parameters.Polymers (Basel). 2025 Aug 1;17(15):2126. doi: 10.3390/polym17152126. Polymers (Basel). 2025. PMID: 40808174 Free PMC article.
-
Role of release modifiers to modulate drug release from fused deposition modelling (FDM) 3D printed tablets.Int J Pharm. 2021 Mar 15;597:120315. doi: 10.1016/j.ijpharm.2021.120315. Epub 2021 Feb 1. Int J Pharm. 2021. PMID: 33540000
-
Exploration of Conventional and FDM-Mediated 3D Printed Tablets Fabricated Using HME-Based Filaments for pH-Dependent Drug Delivery.AAPS PharmSciTech. 2025 Mar 27;26(4):96. doi: 10.1208/s12249-025-03088-6. AAPS PharmSciTech. 2025. PMID: 40148671
-
3D printing in personalized drug delivery: An overview of hot-melt extrusion-based fused deposition modeling.Int J Pharm. 2021 May 1;600:120501. doi: 10.1016/j.ijpharm.2021.120501. Epub 2021 Mar 19. Int J Pharm. 2021. PMID: 33746011 Free PMC article. Review.
Cited by
-
Development of 3D-Printed Bicompartmental Devices by Dual-Nozzle Fused Deposition Modeling (FDM) for Colon-Specific Drug Delivery.Pharmaceutics. 2023 Sep 21;15(9):2362. doi: 10.3390/pharmaceutics15092362. Pharmaceutics. 2023. PMID: 37765330 Free PMC article.
-
Challenges, current status and emerging strategies in the development of rapidly dissolving FDM 3D-printed tablets: An overview and commentary.ADMET DMPK. 2023 Jan 1;11(1):33-55. doi: 10.5599/admet.1622. eCollection 2023. ADMET DMPK. 2023. PMID: 36778904 Free PMC article. Review.
-
Impact of Laser Speed and Drug Particle Size on Selective Laser Sintering 3D Printing of Amorphous Solid Dispersions.Pharmaceutics. 2021 Jul 27;13(8):1149. doi: 10.3390/pharmaceutics13081149. Pharmaceutics. 2021. PMID: 34452109 Free PMC article.
-
3D Printing: Advancement in Biogenerative Engineering to Combat Shortage of Organs and Bioapplicable Materials.Regen Eng Transl Med. 2022;8(2):173-199. doi: 10.1007/s40883-021-00219-w. Epub 2021 Jul 2. Regen Eng Transl Med. 2022. PMID: 34230892 Free PMC article. Review.
-
Exploring 3D Printing in Drug Development: Assessing the Potential of Advanced Melt Drop Deposition Technology for Solubility Enhancement by Creation of Amorphous Solid Dispersions.Pharmaceutics. 2024 Nov 22;16(12):1501. doi: 10.3390/pharmaceutics16121501. Pharmaceutics. 2024. PMID: 39771481 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

