α-Lactalbumin, Amazing Calcium-Binding Protein
- PMID: 32825311
- PMCID: PMC7565966
- DOI: 10.3390/biom10091210
α-Lactalbumin, Amazing Calcium-Binding Protein
Abstract
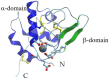
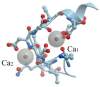
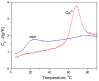
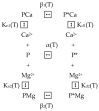
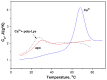
α-Lactalbumin (α-LA) is a small (Mr 14,200), acidic (pI 4-5), Ca2+-binding protein. α-LA is a regulatory component of lactose synthase enzyme system functioning in the lactating mammary gland. The protein possesses a single strong Ca2+-binding site, which can also bind Mg2+, Mn2+, Na+, K+, and some other metal cations. It contains several distinct Zn2+-binding sites. Physical properties of α-LA strongly depend on the occupation of its metal binding sites by metal ions. In the absence of bound metal ions, α-LA is in the molten globule-like state. The binding of metal ions, and especially of Ca2+, increases stability of α-LA against the action of heat, various denaturing agents and proteases, while the binding of Zn2+ to the Ca2+-loaded protein decreases its stability and causes its aggregation. At pH 2, the protein is in the classical molten globule state. α-LA can associate with membranes at neutral or slightly acidic pH at physiological temperatures. Depending on external conditions, α-LA can form amyloid fibrils, amorphous aggregates, nanoparticles, and nanotubes. Some of these aggregated states of α-LA can be used in practical applications such as drug delivery to tissues and organs. α-LA and some of its fragments possess bactericidal and antiviral activities. Complexes of partially unfolded α-LA with oleic acid are cytotoxic to various tumor and bacterial cells. α-LA in the cytotoxic complexes plays a role of a delivery carrier of cytotoxic fatty acid molecules into tumor and bacterial cells across the cell membrane. Perhaps in the future the complexes of α-LA with oleic acid will be used for development of new anti-cancer drugs.
Keywords: amyloid fibrils; cytotoxicity; folding; liprotides; metal binding; molten globule; nanoparticles; nanotubes; structure; unfolding; α-lactalbumin.
Conflict of interest statement
The author declares no conflict of interest.
Figures
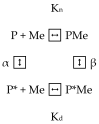
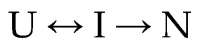
Similar articles
-
Molten globule of bovine alpha-lactalbumin at neutral pH induced by heat, trifluoroethanol, and oleic acid: a comparative analysis by circular dichroism spectroscopy and limited proteolysis.Proteins. 2002 Nov 15;49(3):385-97. doi: 10.1002/prot.10234. Proteins. 2002. PMID: 12360528
-
alpha-Lactalbumin: structure and function.FEBS Lett. 2000 May 19;473(3):269-74. doi: 10.1016/s0014-5793(00)01546-5. FEBS Lett. 2000. PMID: 10818224 Review.
-
α-Lactalbumin forms with oleic acid a high molecular weight complex displaying cytotoxic activity.Biochemistry. 2010 Oct 5;49(39):8658-67. doi: 10.1021/bi1012832. Epub 2010 Sep 9. Biochemistry. 2010. PMID: 20804154
-
Structure and stability analysis of cytotoxic complex of camel α-lactalbumin and unsaturated fatty acids produced at high temperature.J Biomol Struct Dyn. 2011 Jun;28(6):919-28. doi: 10.1080/07391102.2011.10508618. J Biomol Struct Dyn. 2011. PMID: 21469752
-
Disorder in Milk Proteins: α-Lactalbumin. Part B. A Multifunctional Whey Protein Acting as an Oligomeric Molten Globular "Oil Container" in the Anti-Tumorigenic Drugs, Liprotides.Curr Protein Pept Sci. 2016 Jul 15;17(6):612-28. doi: 10.2174/1389203717666151203003151. Curr Protein Pept Sci. 2016. PMID: 26916155 Review.
Cited by
-
Molecular Analytical Assessment of Thermally Precipitated α-Lactalbumin after Resolubilization.Foods. 2021 Sep 20;10(9):2231. doi: 10.3390/foods10092231. Foods. 2021. PMID: 34574341 Free PMC article.
-
Influence of divalent metal cations on α-lactalbumin fibril formation.J Biol Inorg Chem. 2024 Sep;29(6):601-609. doi: 10.1007/s00775-024-02071-z. Epub 2024 Aug 10. J Biol Inorg Chem. 2024. PMID: 39126483
-
Effects of spray drying and freeze drying on the protein profile of whey protein concentrate.J Food Sci. 2024 Nov;89(11):7477-7493. doi: 10.1111/1750-3841.17349. Epub 2024 Oct 4. J Food Sci. 2024. PMID: 39366780
-
Human milk whey proteins: Constituents, influencing factors, detection methods, and comparative analysis with other sources.Food Chem X. 2024 Dec 9;25:102082. doi: 10.1016/j.fochx.2024.102082. eCollection 2025 Jan. Food Chem X. 2024. PMID: 39807410 Free PMC article. Review.
-
Analysis of the mechanisms and efficiency of Taxifolin encapsulation in whey proteins via thermomechanical mixing and spray drying.Food Chem (Oxf). 2025 May 27;10:100261. doi: 10.1016/j.fochms.2025.100261. eCollection 2025 Jun. Food Chem (Oxf). 2025. PMID: 40524876 Free PMC article.
References
-
- Renner E. Milk and Dairy Products in Human Nutrition. John Wiley & Sons; Hoboken, NJ, USA: 1983. Milk and dairy products in human nutrition.
-
- Montagne P., Cuilliere M.L., Mole C., Bene M.C., Faure G. Immunological and nutritional composition of human milk in relation to prematurity and mother’s parity during the first 2 weeks of lactation. J. Pediatric Gastroenterol. Nutr. 1999;29:75–80. doi: 10.1097/00005176-199907000-00018. - DOI - PubMed
-
- Hill R.L., Brew K. Lactose synthetase. Adv. Enzymol. Relat. Areas Mol. Biol. 1975;43:411–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

