Phage Display to Augment Biomaterial Function
- PMID: 32825391
- PMCID: PMC7504225
- DOI: 10.3390/ijms21175994
Phage Display to Augment Biomaterial Function
Abstract
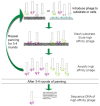
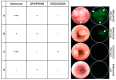
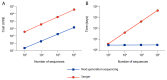
Biomaterial design relies on controlling interactions between materials and their biological environments to modulate the functions of proteins, cells, and tissues. Phage display is a powerful tool that can be used to discover peptide sequences with high affinity for a desired target. When incorporated into biomaterial design, peptides identified via phage display can functionalize material surfaces to control the interaction between a biomaterial and its local microenvironment. A targeting peptide has high specificity for a given target, allowing for homing a specific protein, cell, tissue, or other material to a biomaterial. A functional peptide has an affinity for a given protein, cell, or tissue, but also modulates its target's activity upon binding. Biomaterials can be further enhanced using a combination of targeting and/or functional peptides to create dual-functional peptides for bridging two targets or modulating the behavior of a specific protein or cell. This review will examine current and future applications of phage display for the augmentation of biomaterials.
Keywords: biomaterials; dual-functioning peptides; peptides; phage display; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



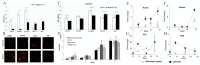
References
-
- Rosenberg A., Griffin K., Studier E.W., Mccormick M., Berg J., Novy R., Mierendorf R. T7 Select phage display system: A powerful new protein display system based on bacteriophage T7. Innovations. 1996;6:1–6.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

