P2X7 Receptors Amplify CNS Damage in Neurodegenerative Diseases
- PMID: 32825423
- PMCID: PMC7504621
- DOI: 10.3390/ijms21175996
P2X7 Receptors Amplify CNS Damage in Neurodegenerative Diseases
Abstract
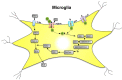
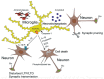
ATP is a (co)transmitter and signaling molecule in the CNS. It acts at a multitude of ligand-gated cationic channels termed P2X to induce rapid depolarization of the cell membrane. Within this receptor-channel family, the P2X7 receptor (R) allows the transmembrane fluxes of Na+, Ca2+, and K+, but also allows the slow permeation of larger organic molecules. This is supposed to cause necrosis by excessive Ca2+ influx, as well as depletion of intracellular ions and metabolites. Cell death may also occur by apoptosis due to the activation of the caspase enzymatic cascade. Because P2X7Rs are localized in the CNS preferentially on microglia, but also at a lower density on neuroglia (astrocytes, oligodendrocytes) the stimulation of this receptor leads to the release of neurodegeneration-inducing bioactive molecules such as pro-inflammatory cytokines, chemokines, proteases, reactive oxygen and nitrogen molecules, and the excitotoxic glutamate/ATP. Various neurodegenerative reactions of the brain/spinal cord following acute harmful events (mechanical CNS damage, ischemia, status epilepticus) or chronic neurodegenerative diseases (neuropathic pain, Alzheimer's disease, Parkinson's disease, multiple sclerosis, amyotrophic lateral sclerosis) lead to a massive release of ATP via the leaky plasma membrane of neural tissue. This causes cellular damage superimposed on the original consequences of neurodegeneration. Hence, blood-brain-barrier permeable pharmacological antagonists of P2X7Rs with excellent bioavailability are possible therapeutic agents for these diseases. The aim of this review article is to summarize our present state of knowledge on the involvement of P2X7R-mediated events in neurodegenerative illnesses endangering especially the life quality and duration of the aged human population.
Keywords: Alzheimer’s disease; P2X7 receptor; Parkinson’s disease; amyotrophic lateral sclerosis; epilepsy; ischemia; mechanical injury; multiple sclerosis; neurodegenerative diseases; neuroinflammation; neuropathic pain.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
P2X7 receptors: channels, pores and more.CNS Neurol Disord Drug Targets. 2012 Sep;11(6):705-21. doi: 10.2174/187152712803581137. CNS Neurol Disord Drug Targets. 2012. PMID: 22963440 Review.
-
P2X7 receptor: an emerging target in central nervous system diseases.Trends Pharmacol Sci. 2014 Oct;35(10):537-47. doi: 10.1016/j.tips.2014.08.002. Epub 2014 Sep 12. Trends Pharmacol Sci. 2014. PMID: 25223574 Review.
-
Ion channels on microglia: therapeutic targets for neuroprotection.CNS Neurol Disord Drug Targets. 2011 Feb;10(1):44-56. doi: 10.2174/187152711794488638. CNS Neurol Disord Drug Targets. 2011. PMID: 21143139 Review.
-
A Possible Causal Involvement of Neuroinflammatory, Purinergic P2X7 Receptors in Psychiatric Disorders.Curr Neuropharmacol. 2022;20(11):2142-2155. doi: 10.2174/1570159X20666220302152400. Curr Neuropharmacol. 2022. PMID: 35236262 Free PMC article.
-
Astrocytic and Oligodendrocytic P2X7 Receptors Determine Neuronal Functions in the CNS.Front Mol Neurosci. 2021 Feb 9;14:641570. doi: 10.3389/fnmol.2021.641570. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33642994 Free PMC article.
Cited by
-
Lacking P2X7-receptors protects substantia nigra dopaminergic neurons and hippocampal-related cognitive performance from the deleterious effects of high-fat diet exposure in adult male mice.Front Nutr. 2024 Jan 25;11:1289750. doi: 10.3389/fnut.2024.1289750. eCollection 2024. Front Nutr. 2024. PMID: 38344021 Free PMC article.
-
Pannexin-1 Channels as Mediators of Neuroinflammation.Int J Mol Sci. 2021 May 14;22(10):5189. doi: 10.3390/ijms22105189. Int J Mol Sci. 2021. PMID: 34068881 Free PMC article. Review.
-
Astroglial and Microglial Purinergic P2X7 Receptor as a Major Contributor to Neuroinflammation during the Course of Multiple Sclerosis.Int J Mol Sci. 2021 Aug 5;22(16):8404. doi: 10.3390/ijms22168404. Int J Mol Sci. 2021. PMID: 34445109 Free PMC article. Review.
-
P2Y1 Receptor as a Catalyst of Brain Neurodegeneration.NeuroSci. 2022 Oct 31;3(4):604-615. doi: 10.3390/neurosci3040043. eCollection 2022 Dec. NeuroSci. 2022. PMID: 39483765 Free PMC article. Review.
-
The Purinergic P2X7 Receptor as a Target for Adjunctive Treatment for Drug-Refractory Epilepsy.Int J Mol Sci. 2024 Jun 23;25(13):6894. doi: 10.3390/ijms25136894. Int J Mol Sci. 2024. PMID: 39000004 Free PMC article. Review.
References
-
- Burnstock G. Purinergic nerves. Pharmacol. Rev. 1972;24:509–581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

