Targeting the Mitochondrial Metabolic Network: A Promising Strategy in Cancer Treatment
- PMID: 32825551
- PMCID: PMC7503725
- DOI: 10.3390/ijms21176014
Targeting the Mitochondrial Metabolic Network: A Promising Strategy in Cancer Treatment
Abstract
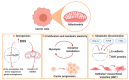
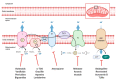
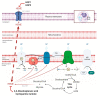
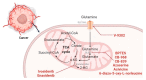
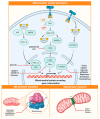
Metabolic reprogramming is a hallmark of cancer, which implements a profound metabolic rewiring in order to support a high proliferation rate and to ensure cell survival in its complex microenvironment. Although initial studies considered glycolysis as a crucial metabolic pathway in tumor metabolism reprogramming (i.e., the Warburg effect), recently, the critical role of mitochondria in oncogenesis, tumor progression, and neoplastic dissemination has emerged. In this report, we examined the main mitochondrial metabolic pathways that are altered in cancer, which play key roles in the different stages of tumor progression. Furthermore, we reviewed the function of important molecules inhibiting the main mitochondrial metabolic processes, which have been proven to be promising anticancer candidates in recent years. In particular, inhibitors of oxidative phosphorylation (OXPHOS), heme flux, the tricarboxylic acid cycle (TCA), glutaminolysis, mitochondrial dynamics, and biogenesis are discussed. The examined mitochondrial metabolic network inhibitors have produced interesting results in both preclinical and clinical studies, advancing cancer research and emphasizing that mitochondrial targeting may represent an effective anticancer strategy.
Keywords: cancer therapy; metabolic network; metabolic rewiring; mitochondria; targeting mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

