A First Insight into the Structural and Functional Comparison of Environmental Microbiota in Freshwater Turtle Chinemys reevesii at Different Growth Stages under Pond and Greenhouse Cultivation
- PMID: 32825672
- PMCID: PMC7564371
- DOI: 10.3390/microorganisms8091277
A First Insight into the Structural and Functional Comparison of Environmental Microbiota in Freshwater Turtle Chinemys reevesii at Different Growth Stages under Pond and Greenhouse Cultivation
Abstract
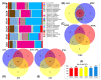
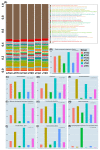
The microbial community structure of water is an important indicator for evaluating the water quality of the aquaculture environment. In this study, the investigation and comparison of the bacterial communities of pond cultivation (PC) and greenhouse cultivation (GC) between hatchling, juvenile, and adult growth stages of C. reevesii were performed. In addition, the V4 regions of the 16S rRNA gene were sequenced. The Chao1 richness estimator of the PC group was significantly higher than that of the GC group. The beta diversity showed that the microbiotas of the two groups were isolated from each other. The dominant phyla were Cyanobacteria, Proteobacteria, Actinobacteria, Bacteroidetes, Verrucomicrobia, and Planctomycetes in the PC group and Proteobacteria, Bacteroidetes, Firmicutes, Cyanobacteria, Chloroflexi, and Actinobacteria in the GC group. Both the numbers and the types of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotations differed between the PC and GC groups. The prediction of bacterial phenotype implied that the GC environment is more likely to deteriorate, and turtles are more susceptible to pathogens than those of the PC environment. In addition, a total of nine potential pathogenic bacteria were identified and the correlation of environmental factors analyses showed significant differences of bacterial species between the PC and GC groups, while the potential pathogenic bacteria showed significant correlation with the stocking density, temperature, pH, orthophosphate (PO4-P), and dissolved oxygen (DO) in both the PC and GC groups. Noticeably, this is the first report to describe the different microbiota characteristics of the different cultivation environments in the different growth stages of C. reevesii, which will provide valuable data for water quality adjustment, disease prevention, and the healthy breeding of turtles.
Keywords: Chinemys reevesii; different growth stages; environmental microbiota; greenhouse cultivation; pond cultivation.
Conflict of interest statement
The authors report no conflict of interest. The authors alone are responsible for the content and writing of this article.
Figures




Similar articles
-
Variations in the Intestinal Microbiota of the Chinese Soft-Shelled Turtle (Trionyx sinensis) between Greenhouse and Pond Aquaculture.Animals (Basel). 2023 Sep 20;13(18):2971. doi: 10.3390/ani13182971. Animals (Basel). 2023. PMID: 37760371 Free PMC article.
-
The invasive red-eared slider turtle is more successful than the native Chinese three-keeled pond turtle: evidence from the gut microbiota.PeerJ. 2020 Oct 29;8:e10271. doi: 10.7717/peerj.10271. eCollection 2020. PeerJ. 2020. PMID: 33194431 Free PMC article.
-
Microbiota comparison of Pacific white shrimp intestine and sediment at freshwater and marine cultured environment.Sci Total Environ. 2019 Mar 20;657:1194-1204. doi: 10.1016/j.scitotenv.2018.12.069. Epub 2018 Dec 6. Sci Total Environ. 2019. PMID: 30677886
-
Metagenomics Analysis Reveals the Composition and Functional Differences of Fecal Microbiota in Wild, Farm, and Released Chinese Three-Keeled Pond Turtles (Mauremys reevesii).Animals (Basel). 2024 Jun 10;14(12):1750. doi: 10.3390/ani14121750. Animals (Basel). 2024. PMID: 38929370 Free PMC article.
-
Gut microbiota of homologous Chinese soft-shell turtles (Pelodiscus sinensis) in different habitats.BMC Microbiol. 2021 May 11;21(1):142. doi: 10.1186/s12866-021-02209-y. BMC Microbiol. 2021. PMID: 33975559 Free PMC article.
Cited by
-
Fine-scale geographic difference of the endangered Big-headed Turtle (Platysternon megacephalum) fecal microbiota, and comparison with the syntopic Beale's Eyed Turtle (Sacalia bealei).BMC Microbiol. 2024 Feb 29;24(1):71. doi: 10.1186/s12866-024-03227-2. BMC Microbiol. 2024. PMID: 38418973 Free PMC article.
References
-
- Chakroff M. Freshwater Fish Pond Culture and Management. VITA. Publications; Arlington, VA, USA: 1976.
-
- Sarkar B., Tiwari G.N. Thermal modeling of a greenhouse fish pond system. Agric. Eng. Int. CIGR J. 2005;7:1–18.
-
- Borowitzka M.A., Moheimani N.R. Algae for Biofuels and Energy. Springer; Dordrecht, The Netherlands: 2013. Open Pond Culture Systems; pp. 133–152.
-
- Cleveland R., Norris R.D., Mace I. Proceedings of the AGU Fall Meeting 2019. American Geophysical Union; Washington, DC, USA: 2019. Global Fish Production in the Cenozoic Greenhouse and Icehouse Oceans.
-
- Peng Y.R. The development history and training algorithm of artificial neuralnetwork. Public Commun. Sci. Technol. 2018;10:129–130.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

