Anti-Influenza Protective Efficacy of a H6 Virus-Like Particle in Chickens
- PMID: 32825685
- PMCID: PMC7565593
- DOI: 10.3390/vaccines8030465
Anti-Influenza Protective Efficacy of a H6 Virus-Like Particle in Chickens
Abstract
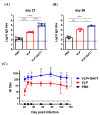
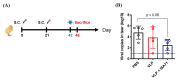
H6 avian influenza viruses (AIVs) have a worldwide distribution, and they pose a potential concern for public health. In Taiwan, H6 AIVs have circulated in domestic chickens for more than 40 years, and certain strains have crossed the species barrier to infect mammals. With the goal of containing the disease, there is a pressing need to develop a safe and effective vaccine for pandemic preparedness. In this study, we prepared a virus-like particle (VLP) that consisted of the hemagglutinin (HA) and matrix protein 1 (M1) derived from a H6 AIV as a vaccine antigen, and we examined the immunogenicity and protective efficacy when combined with an adjuvant in a chicken model. Full-length HA and M1 protein genes were cloned and expressed using a baculovirus expression system, and VLPs were purified from the supernatant of insect cell cultures. We performed nanoparticle-tracking analysis and transmission electron microscopy to validate that the particle structure and properties resembled the native virions. In animal experiments, specific-pathogen-free chickens that received the H6 VLPs in combination with an adjuvant showed superior H6N1 virus-specific serum IgG and hemagglutination-inhibition antibody responses, which lasted more than 112 days. Following the H6N1 viral challenge, the vaccinated chickens showed reduced viral replication in the lungs, kidneys and conjunctival/cloacal shedding. The antibodies induced in the chickens by the vaccine were able to cross-react with the H6N1 human isolate and drifted avian H6N1 isolates. In summary, the H6 VLP vaccine elicited superb immunogenicity in vivo, and the use of an adjuvant further enhanced the antiviral protective efficacy. This vaccine formulation could potentially be used to manage H6 influenza virus infections in chickens.
Keywords: H6 avian influenza virus; adjuvant; hemagglutinin; matrix 1; vaccine; virus-like particles.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Munster V.J., Baas C., Lexmond P., Waldenström J., Wallensten A., Fransson T., Rimmelzwaan G.F., Beyer W.E., Schutten M., Olsen B., et al. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog. 2007;3:e61. doi: 10.1371/journal.ppat.0030061. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

