Transition Metal Complexes with Flufenamic Acid for Pharmaceutical Applications-A Novel Three-Centered Coordination Polymer of Mn(II) Flufenamate
- PMID: 32825746
- PMCID: PMC7503579
- DOI: 10.3390/ma13173705
Transition Metal Complexes with Flufenamic Acid for Pharmaceutical Applications-A Novel Three-Centered Coordination Polymer of Mn(II) Flufenamate
Abstract
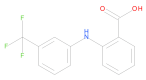
Five complexes of Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) with non-steroidal anti-inflammatory drug, flufenamic acid were synthesized: (1) [Mn3(fluf)6EtOH)(H2O)]·3EtOH; (2) [Co(fluf)2(EtOH)(H2O)]·H2O; (3) [Ni(fluf)2(EtOH)(H2O)]·H2O; (4) [Cu(fluf)2·H2O]; (5) [Zn(fluf)2·H2O]. All complexes were characterized by elemental analysis (EA), flame atomic absorption spectrometry (FAAS), Fourier-transform infrared spectroscopy (FTIR), and thermogravimetric analysis (TGA). The crystal structure of 1 was determined by the single crystal X-ray diffraction technique. It crystallizes in the triclinic space group P with three independent Mn(II) cations, six coordinated flufenamato ligands augmented with water and ethanol molecules in the inner coordination sphere. In this crystal, manganese atoms are multiplied by symmetry and form infinite, polymeric chains which extend along the [001] dimension. The Hirshfeld Surface analysis revealed changes in interaction assemblies around all metal centers. The antioxidant and antimicrobial activities were established for all complexes and free ligand for comparison. All compounds exhibit good or moderate bioactivity against Gram-positive bacteria and yeasts.
Keywords: antioxidant activity; coordination polymer; flufenamic acid; non-steroidal anti-inflammatory drug-complex; thermal analysis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Bagherzadeh M., Amini M., Boghaei D.M., Najafpour M.M., McKee V. Synthesis, X–ray structure, characterization and catalytic activity of a polymeric manganese(II) complex with iminodiacetate. Appl. Organomet. Chem. 2011;25:559–563. doi: 10.1002/aoc.1802. - DOI
-
- Keggin J.F., Miles F.D. Structures and formulæ of the prussian blues and related compounds. Nature. 1936;137:577–578. doi: 10.1038/137577a0. - DOI
-
- Ghasdian N., Liu Y., McHale R., He J., Miao Y., Wang X. Synthesis of Prussian Blue Metal Coordination Polymer Nanocubes via Cyanoferrate Monomer Design. J. Inorg. Organomet. Polym. Mater. 2013;23:111–118. doi: 10.1007/s10904-012-9748-y. - DOI
-
- Donaruma L.G., Block B.P., Loening K.L., Plate N., Tsuruta T., Buschbeck K.C., Powell W.H., Reedijk J. Nomenclature for regular single-strand and quasi single-strand inorganic and coordination polymers. Pure Appl. Chem. 1985;57:149–168.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

