Heterogeneity and clonal relationships of adaptive immune cells in ulcerative colitis revealed by single-cell analyses
- PMID: 32826341
- PMCID: PMC7733868
- DOI: 10.1126/sciimmunol.abb4432
Heterogeneity and clonal relationships of adaptive immune cells in ulcerative colitis revealed by single-cell analyses
Abstract
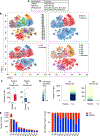
Inflammatory bowel disease (IBD) encompasses a spectrum of gastrointestinal disorders driven by dysregulated immune responses against gut microbiota. We integrated single-cell RNA and antigen receptor sequencing to elucidate key components, cellular states, and clonal relationships of the peripheral and gastrointestinal mucosal immune systems in health and ulcerative colitis (UC). UC was associated with an increase in IgG1+ plasma cells in colonic tissue, increased colonic regulatory T cells characterized by elevated expression of the transcription factor ZEB2, and an enrichment of a γδ T cell subset in the peripheral blood. Moreover, we observed heterogeneity in CD8+ tissue-resident memory T (TRM) cells in colonic tissue, with four transcriptionally distinct states of differentiation observed across health and disease. In the setting of UC, there was a marked shift of clonally related CD8+ TRM cells toward an inflammatory state, mediated, in part, by increased expression of the T-box transcription factor Eomesodermin. Together, these results provide a detailed atlas of transcriptional changes occurring in adaptive immune cells in the context of UC and suggest a role for CD8+ TRM cells in IBD.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures







References
-
- Na YR, Stakenborg M, Seok SH, Matteoli G, Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nature reviews. Gastroenterology & hepatology 16, 531–543 (2019). - PubMed
-
- Bal SM, Golebski K, Spits H, Plasticity of innate lymphoid cell subsets. Nature reviews. Immunology, (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI100880/AI/NIAID NIH HHS/United States
- R01 AI123202/AI/NIAID NIH HHS/United States
- R01 AI129973/AI/NIAID NIH HHS/United States
- P30 DK120515/DK/NIDDK NIH HHS/United States
- KL2 TR001444/TR/NCATS NIH HHS/United States
- I01 BX003424/BX/BLRD VA/United States
- S10 OD026929/OD/NIH HHS/United States
- T32 DK007202/DK/NIDDK NIH HHS/United States
- P30 CA023100/CA/NCI NIH HHS/United States
- R37 AI067545/AI/NIAID NIH HHS/United States
- Z01 AI000880/ImNIH/Intramural NIH HHS/United States
- U19 MH107367/MH/NIMH NIH HHS/United States
- R21 DK119724/DK/NIDDK NIH HHS/United States
- P30 NS047101/NS/NINDS NIH HHS/United States
- R01 AI082850/AI/NIAID NIH HHS/United States
- S10 OD018499/OD/NIH HHS/United States
- K23 DK117058/DK/NIDDK NIH HHS/United States
- P01 AI132122/AI/NIAID NIH HHS/United States
- K23 DK123406/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

