Immunometabolism in the pathogenesis of systemic lupus erythematosus: an update
- PMID: 32826478
- PMCID: PMC10463177
- DOI: 10.1097/BOR.0000000000000738
Immunometabolism in the pathogenesis of systemic lupus erythematosus: an update
Abstract
Purpose of review: To provide an update on state-of-the-art evidence on the role of immunometabolism reprogramming in the pathogenesis of systemic lupus erythematosus (SLE).
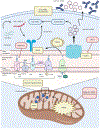
Recent findings: Mitochondrial dysfunction and enhanced oxidative stress, along with specific defects in other metabolic pathways, can promote dysregulation of innate and adaptive immune responses in SLE. These abnormalities appear to be driven by genetic and epigenetic factors, modulated by stochastic events. In addition to extensive descriptions of abnormalities in immunometabolism of lupus lymphocytes, recent studies support the critical role of dysregulation of metabolic pathways in innate immune cells including neutrophils, macrophages and dendritic cells, in SLE pathogenesis. Recent abnormalities described in lipid metabolism have been associated with SLE disease activity and related damage. Promising therapeutic strategies that target these metabolic abnormalities have recently been described in SLE.
Summary: Fundamental new insights regarding the role of mitochondrial dysfunction in innate immune dysregulation in SLE pathogenesis have recently emerged. Defects in specific molecular pathways pertinent to immunometabolism in SLE have been described. New insights in translational medicine and promising therapeutic targets have been proposed based on these recent findings.
Conflict of interest statement
Conflicts of interest
There are no conflicts of interest.
Figures

Similar articles
-
Innate Immune Dysregulation in the Development of Cardiovascular Disease in Lupus.Curr Rheumatol Rep. 2019 Jul 23;21(9):46. doi: 10.1007/s11926-019-0842-9. Curr Rheumatol Rep. 2019. PMID: 31338604 Review.
-
[Immunometabolism and systemic lupus erythematosus].Beijing Da Xue Xue Bao Yi Xue Ban. 2018 Dec 18;50(6):1120-1124. Beijing Da Xue Xue Bao Yi Xue Ban. 2018. PMID: 30562794 Chinese.
-
Neutrophils in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus: Same Foe Different M.O.Front Immunol. 2021 Mar 4;12:649693. doi: 10.3389/fimmu.2021.649693. eCollection 2021. Front Immunol. 2021. PMID: 33746988 Free PMC article. Review.
-
Metabolic abnormalities and oxidative stress in lupus.Curr Opin Rheumatol. 2017 Sep;29(5):442-449. doi: 10.1097/BOR.0000000000000413. Curr Opin Rheumatol. 2017. PMID: 28639951 Free PMC article. Review.
-
Macrophages and neutrophils in SLE-An online molecular catalog.Autoimmun Rev. 2012 Mar;11(5):365-72. doi: 10.1016/j.autrev.2011.10.010. Epub 2011 Oct 19. Autoimmun Rev. 2012. PMID: 22036828 Review.
Cited by
-
Expression and Significance of Programmed Death-1 and Its Ligands in the Accelerated Formation of Atherosclerosis in an Induced Murine Lupus Model.Biomed Res Int. 2022 Nov 16;2022:6255383. doi: 10.1155/2022/6255383. eCollection 2022. Biomed Res Int. 2022. PMID: 39050559 Free PMC article.
-
Neutrophil glucose flux as a therapeutic target in antiphospholipid syndrome.J Clin Invest. 2024 Jun 13;134(15):e169893. doi: 10.1172/JCI169893. J Clin Invest. 2024. PMID: 38869951 Free PMC article.
-
The Aconitate Decarboxylase 1/Itaconate Pathway Modulates Immune Dysregulation and Associates with Cardiovascular Disease Markers and Disease Activity in Systemic Lupus Erythematosus.J Immunol. 2024 Aug 15;213(4):419-434. doi: 10.4049/jimmunol.2400241. J Immunol. 2024. PMID: 38949522 Free PMC article.
-
Modulation of the Itaconate Pathway Attenuates Murine Lupus.Arthritis Rheumatol. 2022 Dec;74(12):1971-1983. doi: 10.1002/art.42284. Epub 2022 Nov 6. Arthritis Rheumatol. 2022. PMID: 35791960 Free PMC article.
-
Serum amyloid A-to-albumin ratio as a potential biomarker to predict the activity, severity, and poor prognosis of systemic lupus erythematosus.J Clin Lab Anal. 2022 Mar;36(3):e24282. doi: 10.1002/jcla.24282. Epub 2022 Feb 10. J Clin Lab Anal. 2022. PMID: 35141936 Free PMC article.
References
-
- Stathopoulou C, Nikoleri D, Bertsias G. Immunometabolism: an overview and therapeutic prospects in autoimmune diseases. Immunotherapy. 2019;11(9):813–29. - PubMed
-
- Morel L Immunometabolism in systemic lupus erythematosus. Nat Rev Rheumatol. 2017;13(5):280–90. - PubMed
-
- Xiao YB, Guo MY, Zuo XX. [Immunometabolism and systemic lupus erythematosus]. Beijing Da Xue Xue Bao Yi Xue Ban. 2018;50(6):1120–4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

