Pooled Efficacy and Safety Profile of Netarsudil Ophthalmic Solution 0.02% in Patients With Open-angle Glaucoma or Ocular Hypertension
- PMID: 32826769
- PMCID: PMC7647436
- DOI: 10.1097/IJG.0000000000001634
Pooled Efficacy and Safety Profile of Netarsudil Ophthalmic Solution 0.02% in Patients With Open-angle Glaucoma or Ocular Hypertension
Abstract
Precis: In pooled phase III analyses, once-daily netarsudil 0.02% resulted in intraocular pressure (IOP) reduction that was noninferior to twice-daily timolol 0.5%, with minimal treatment-related serious or systemic adverse events (AEs). Ocular AEs were generally tolerable.
Purpose: The purpose of this study was to assess the efficacy and safety of the Rho kinase inhibitor netarsudil in patients with open-angle glaucoma or ocular hypertension.
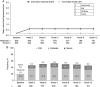
Patients and methods: Pooled analysis of data from the ROCKET-1 to 4 phase III studies of once-daily (PM) netarsudil or twice-daily timolol in patients with open-angle glaucoma or ocular hypertension. The primary efficacy measure was mean IOP at 8:00 AM, 10:00 AM, and 4:00 PM at week 2, week 6, and month 3 in patients with baseline IOP <25 mm Hg.
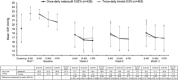
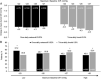
Results: In the pooled primary efficacy population (netarsudil, n=494; timolol, n=510), once-daily netarsudil was noninferior to twice-daily timolol at all 9 timepoints through month 3. Mean treated IOP ranged from 16.4 to 18.1 mm Hg among netarsudil-treated patients and 16.8 to 17.6 mm Hg among timolol-treated patients. In the pooled safety population (n=839 in each treatment group), treatment-related serious AEs occurred at similar frequencies in each treatment group (netarsudil, 0.1%; timolol, 0%). The most common ocular AE, conjunctival hyperemia (netarsudil, 54.4%; timolol, 10.4%), was graded as mild in 77.6% (354/456) of affected netarsudil-treated patients.
Conclusions: Once-daily netarsudil resulted in IOP lowering that was noninferior to twice-daily timolol, with tolerable ocular AEs that were generally mild and self-resolving. As a first-in-class agent in the United States, with a novel mechanism of action, netarsudil may provide a useful therapeutic option for patients who would benefit from IOP lowering.
Conflict of interest statement
Disclosure: I.P.S. is a paid consultant to Aerie Pharmaceuticals and has received research funding from Aerie Pharmaceuticals, Ivantis, Glaukos, Bausch + Lomb/Valeant, Allergan, Ellex, Alcon, and Katena/IOP. He has also served as consultant to Glaukos, Bausch + Lomb/Valeant, Allergan, and Ellex and on speakers’ bureaus for Glaukos, Bausch + Lomb/Valeant, Allergan, Ellex, Alcon, Katena/IOP, Shire, and Novabay. R.D.F. is a paid consultant of Aerie Pharmaceuticals and has served as a consultant to Akorn, Nicox, and Novartis. His research is supported, in part, by an unrestricted grant from Research to Prevent Blindness. J.S.M. has received honoraria from Allergan and Novartis and serves as a consultant to Aerie Pharmaceuticals, Allergan, Glaukos, MicrOptx, and Olleyes. His institution has received research grants from Aerie Pharmaceuticals, Allergan, Bausch + Lomb/Valeant, Diopsys, Heidelberg, Inotek, Merck, Novartis, Olleyes, and Zeiss. T.K. serves as a consultant to, and his institution has received research funding from, Aerie Pharmaceuticals. H.M., R.A.L., and C.C.K. are salaried employees of and own stock in Aerie Pharmaceuticals. D.W.U. is a paid statistical consultant to Aerie Pharmaceuticals. H.S. and T.H. are previous employees of Aerie Pharmaceuticals.
Figures


References
-
- Vrabec JP, Levin LA. The neurobiology of cell death in glaucoma. Eye. 2007;21(suppl 1):S11–S14. - PubMed
-
- Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. - PubMed
-
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:498–505. - PubMed
-
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

