Role of adhesion forces in mechanosensitive channel gating in Staphylococcus aureus adhering to surfaces
- PMID: 32826897
- PMCID: PMC7442641
- DOI: 10.1038/s41522-020-00141-z
Role of adhesion forces in mechanosensitive channel gating in Staphylococcus aureus adhering to surfaces
Abstract
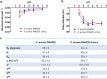
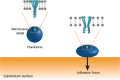
Mechanosensitive channels in bacterial membranes open or close in response to environmental changes to allow transmembrane transport, including antibiotic uptake and solute efflux. In this paper, we hypothesize that gating of mechanosensitive channels is stimulated by forces through which bacteria adhere to surfaces. Hereto, channel gating is related with adhesion forces to different surfaces of a Staphylococcus aureus strain and its isogenic ΔmscL mutant, deficient in MscL (large) channel gating. Staphylococci becoming fluorescent due to uptake of calcein, increased with adhesion force and were higher in the parent strain (66% when adhering with an adhesion force above 4.0 nN) than in the ΔmscL mutant (40% above 1.2 nN). This suggests that MscL channels open at a higher critical adhesion force than at which physically different, MscS (small) channels open and contribute to transmembrane transport. Uptake of the antibiotic dihydrostreptomycin was monitored by staphylococcal killing. The parent strain exposed to dihydrostreptomycin yielded a CFU reduction of 2.3 log-units when adhering with an adhesion force above 3.5 nN, but CFU reduction remained low (1.0 log-unit) in the mutant, independent of adhesion force. This confirms that large channels open at a higher critical adhesion-force than small channels, as also concluded from calcein transmembrane transport. Collectively, these observations support our hypothesis that adhesion forces to surfaces play an important role, next to other established driving forces, in staphylococcal channel gating. This provides an interesting extension of our understanding of transmembrane antibiotic uptake and solute efflux in infectious staphylococcal biofilms in which bacteria experience adhesion forces from a wide variety of surfaces, like those of other bacteria, tissue cells, or implanted biomaterials.
Conflict of interest statement
H.J.B. is also director of a consulting company, SASA BV. The authors declare no potential conflicts of interest with respect to authorship and/or publication of this article. Opinions and assertions contained herein are those of the authors and are not construed as necessarily representing views of their respective employers.
Figures


Similar articles
-
Adhesion force sensing and activation of a membrane-bound sensor to activate nisin efflux pumps in Staphylococcus aureus under mechanical and chemical stresses.J Colloid Interface Sci. 2018 Feb 15;512:14-20. doi: 10.1016/j.jcis.2017.10.024. Epub 2017 Oct 7. J Colloid Interface Sci. 2018. PMID: 29054003
-
Emergent Properties in Streptococcus mutans Biofilms Are Controlled through Adhesion Force Sensing by Initial Colonizers.mBio. 2019 Sep 10;10(5):e01908-19. doi: 10.1128/mBio.01908-19. mBio. 2019. PMID: 31506311 Free PMC article.
-
Influence of Adhesion Force on icaA and cidA Gene Expression and Production of Matrix Components in Staphylococcus aureus Biofilms.Appl Environ Microbiol. 2015 May 15;81(10):3369-78. doi: 10.1128/AEM.04178-14. Epub 2015 Mar 6. Appl Environ Microbiol. 2015. PMID: 25746995 Free PMC article.
-
From membrane tension to channel gating: A principal energy transfer mechanism for mechanosensitive channels.Protein Sci. 2016 Nov;25(11):1954-1964. doi: 10.1002/pro.3017. Epub 2016 Aug 23. Protein Sci. 2016. PMID: 27530280 Free PMC article. Review.
-
How ion channels sense mechanical force: insights from mechanosensitive K2P channels TRAAK, TREK1, and TREK2.Ann N Y Acad Sci. 2015 Sep;1352:20-32. doi: 10.1111/nyas.12874. Epub 2015 Aug 31. Ann N Y Acad Sci. 2015. PMID: 26332952 Review.
Cited by
-
"Force-From-Lipids" Dependence of the MscCG Mechanosensitive Channel Gating on Anionic Membranes.Microorganisms. 2023 Jan 12;11(1):194. doi: 10.3390/microorganisms11010194. Microorganisms. 2023. PMID: 36677485 Free PMC article.
-
Bacterial Virulence and Prevention for Human Spaceflight.Life (Basel). 2023 Feb 27;13(3):656. doi: 10.3390/life13030656. Life (Basel). 2023. PMID: 36983812 Free PMC article.
-
LubriShieldTM-A permanent urinary catheter coating that prevents uropathogen biofilm formation in vitro independent of host protein conditioning.PLoS One. 2025 Jul 10;20(7):e0328167. doi: 10.1371/journal.pone.0328167. eCollection 2025. PLoS One. 2025. PMID: 40638652 Free PMC article.
-
Bacterial-host adhesion dominated by collagen subtypes remodelled by osmotic pressure.NPJ Biofilms Microbiomes. 2024 Nov 12;10(1):124. doi: 10.1038/s41522-024-00600-x. NPJ Biofilms Microbiomes. 2024. PMID: 39532878 Free PMC article.
-
A Comparison of the Adaptive Response of Staphylococcus aureus vs. Streptococcus mutans and the Development of Chlorhexidine Resistance.Front Microbiol. 2022 May 19;13:861890. doi: 10.3389/fmicb.2022.861890. eCollection 2022. Front Microbiol. 2022. PMID: 35694293 Free PMC article.
References
-
- Cox CD, Bavi N, Martinac B. Bacterial mechanosensors. Annu. Rev. Physiol. 2018;80:71–93. - PubMed
-
- Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 2002;9:696–703. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

