Fabrication of multifunctional TANI/Cu2O/Ag nanocomposite for environmental abatement
- PMID: 32826928
- PMCID: PMC7442793
- DOI: 10.1038/s41598-020-70194-9
Fabrication of multifunctional TANI/Cu2O/Ag nanocomposite for environmental abatement
Abstract
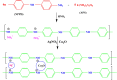
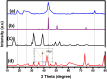
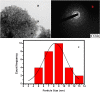
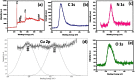
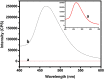
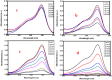
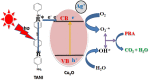
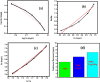
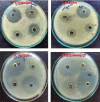
During past decade, the ternary nanocomposite is ubiquitous in nanotechnology. Herein, a simple fabrication of cuprous oxide (Cu2O) and silver (Ag) nanoparticles into Tetraaniline (TANI) matrix by in situ-polymerization approach to attain Tetramer-Metal Oxide-Metal (TANI/Cu2O/Ag, shortly TCA) ternary composite was reported firstly. The synthesized materials were further characterized by a series of instrumental techniques to understand its structure, morphology and thermal properties. This nanocomposite showed promising applications in wastewater treatment by the testing of photocatalytic activity over the pararosaniline hydrochloride (PRA) dye degradation under visible light radiations, removal of Cadmium ion (Cd2+) by adsorption, corrosion resistance and antibacterial activity against both gram positive and gram negative bacterial strains. The obtained results of TCA compared with the pure TANI and binary nanocomposite (TANI/Cu2O) declared that the TCA composite is excellent material to solve the environmental issues due to lesser bandgap energy, visible light respond, high absorptivity, and long-life excitation.
Conflict of interest statement
The authors declare no competing interest.
Figures





References
-
- Speidel EDH, Ruedisili LC, Agnew AF. Perspectives on Water Uses and Abuses. New York: Oxford University Press; 1988.
-
- United State Environmental Protection Agency (EPA). The National Water Quality Inventory: Report to Congress for the 2002 Reporting Cycle—a Profile, Washington DC. Facts Sheet No. EPA 841-F-07- 03 (2007).
-
- Lagaly G. Pesticide–clay inte ractions and formulations. Appl. Clay Sci. 2001;18:205–209.
-
- Fan Z, Yue-Hua L, Jing-Yu L, Zi-Rong T, Yi-Jun X. 3D graphene-based gel photocatalysts for environmental pollutants degradation. Environ. Pollut. 2019;253:365–376. - PubMed
-
- Xin S, Liu JG, Chen Q, Xiao Z, Zhang G, Xin Y. Electricity generation and microbial community of single-chamber microbial fuel cells in response to Cu2O nanoparticles/reduced graphene oxide as cathode catalyst. Chem. Eng. J. 2020;380:122446.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

