TGF-β is insufficient to induce adipocyte state loss without concurrent PPARγ downregulation
- PMID: 32826933
- PMCID: PMC7442643
- DOI: 10.1038/s41598-020-71100-z
TGF-β is insufficient to induce adipocyte state loss without concurrent PPARγ downregulation
Abstract
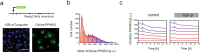
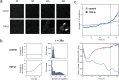
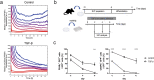
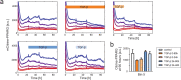
Cell plasticity, the ability of differentiated cells to convert into other cell types, underlies the pathogenesis of many diseases including the transdifferentiation of adipocytes (fat cells) into myofibroblasts in the pathogenesis of dermal fibrosis. Loss of adipocyte identity is an early step in different types of adipocyte plasticity. In this study, we determine the dynamics of adipocyte state loss in response to the profibrotic cytokine TGF-β. We use two complementary approaches, lineage tracing and live fluorescent microscopy, which both allow for robust quantitative tracking of adipocyte identity loss at the single-cell level. We find that the intracellular TGF-β signaling in adipocytes is inhibited by the transcriptional factor PPARγ, specifically by its ubiquitously expressed isoform PPARγ1. However, TGF-β can lead to adipocyte state loss when it is present simultaneously with another stimulus. Our findings establish that an integration of stimuli occurring in a specific order is pivotal for adipocyte state loss which underlies adipocyte plasticity. Our results also suggest the possibility of a more general switch-like mechanism between adipogenic and profibrotic molecular states.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

