Real-world effectiveness of rotavirus vaccines, 2006-19: a literature review and meta-analysis
- PMID: 32827481
- PMCID: PMC8097518
- DOI: 10.1016/S2214-109X(20)30262-X
Real-world effectiveness of rotavirus vaccines, 2006-19: a literature review and meta-analysis
Abstract
Background: Since licensure in 2006, rotavirus vaccines have been introduced in more than 100 countries. The efficacy of rotavirus vaccines is variable in settings with different child mortality levels. We did an updated review of the published literature to assess the real-world effectiveness of rotavirus vaccines in a range of settings.
Methods: In this literature review and meta-analysis, we included observational, post-licensure studies of rotavirus vaccines, published from Jan 1, 2006, to Dec 31, 2019, in English, with laboratory-confirmed rotavirus as the endpoint. In addition to product-specific results for Rotarix (GlaxoSmithKline Biologicals, Rixensart, Belgium) or RotaTeq (Merck, West Point, PA, USA), we included Rotarix and RotaTeq mixed series, and non-product-specific vaccine effectiveness estimates from countries where Rotarix and RotaTeq are both available. Studies of other infant rotavirus vaccines were excluded because little or no post-licensure data were available. We fitted random-effects regression models to estimate vaccine effectiveness among children younger than 12 months and aged 12-23 months. On the basis of 2017 UNICEF mortality estimates for children younger than 5 years, countries were stratified as having low (lowest quartile), medium (second quartile), or high mortality (third and fourth quartiles).
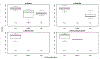
Findings: We identified and screened 1703 articles, of which 60 studies from 32 countries were included. 31 studies were from countries with low child mortality, eight were from medium-mortality countries, and 21 were from high-mortality countries. Rotarix vaccine effectiveness against laboratory-confirmed rotavirus among children younger than 12 months old was 86% (95% CI 81-90) in low-mortality countries, 77% (66-85) in medium-mortality countries, and 63% (54-70) in high-mortality countries. Rotarix vaccine effectiveness among children aged 12-23 months was 86% (81-90) in low-mortality countries, 54% (23-73) in medium-mortality countries, and 58% (38-72) in high-mortality countries. RotaTeq vaccine effectiveness among children younger than 12 months was 86% (76-92) in low-mortality countries and 66% (51-76) in high-mortality countries. RotaTeq vaccine effectiveness among children aged 12-23 months was 84% (79-89) in low-mortality countries. There was no substantial heterogeneity (I2 range: 0-36%). Median vaccine effectiveness in low-mortality countries was similar for Rotarix (83%; IQR 78-91), RotaTeq (85%; 81-92), mixed series (86%; 70-91), and non-product-specific (89%; 75-91) vaccination.
Interpretation: Rotavirus vaccines were effective in preventing rotavirus diarrhoea, with higher performance in countries with lower child mortality.
Funding: None.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Figures



References
-
- Vaccine in National Immunization Programme Update. In: Immunization VaB, editor. https://www.who.int/immunization/monitoring_surveillance/en/: World Health Organization; 2020.
-
- Rotavirus vaccines. WHO position paper - January 2013. Wkly Epidemiol Rec 2013; 88(5): 49–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

