Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial
- PMID: 32828825
- PMCID: PMC10649377
- DOI: 10.1016/j.annonc.2020.08.2098
Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial
Abstract
Background: In EMBRACA, talazoparib prolonged progression-free survival versus chemotherapy (hazard ratio [HR] 0.542 [95% confidence interval (CI) 0.413-0.711]; P < 0.0001) and improved patient-reported outcomes (PRO) in germline BRCA1/2 (gBRCA1/2)-mutated advanced breast cancer (ABC). We report final overall survival (OS).
Patients and methods: This randomized phase III trial enrolled patients with gBRCA1/2-mutated HER2-negative ABC. Patients received talazoparib or physician's choice of chemotherapy. OS was analyzed using stratified HR and log-rank test and prespecified rank-preserving structural failure time model to account for subsequent treatments.
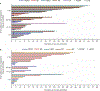
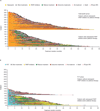
Results: A total of 431 patients were entered in a randomized study (287 talazoparib/144 chemotherapy) with 412 patients treated (286 talazoparib/126 chemotherapy). By 30 September 2019, 216 deaths (75.3%) occurred for talazoparib and 108 (75.0%) chemotherapy; median follow-up was 44.9 and 36.8 months, respectively. HR for OS with talazoparib versus chemotherapy was 0.848 (95% CI 0.670-1.073; P = 0.17); median (95% CI) 19.3 months (16.6-22.5 months) versus 19.5 months (17.4-22.4 months). Kaplan-Meier survival percentages (95% CI) for talazoparib versus chemotherapy: month 12, 71% (66% to 76%)/74% (66% to 81%); month 24, 42% (36% to 47%)/38% (30% to 47%); month 36, 27% (22% to 33%)/21% (14% to 29%). Most patients received subsequent treatments: for talazoparib and chemotherapy, 46.3%/41.7% received platinum and 4.5%/32.6% received a poly(ADP-ribose) polymerase (PARP) inhibitor, respectively. Adjusting for subsequent PARP and/or platinum use, HR for OS was 0.756 (95% bootstrap CI 0.503-1.029). Grade 3-4 adverse events occurred in 69.6% (talazoparib) and 64.3% (chemotherapy) patients, consistent with previous reports. Extended follow-up showed significant overall improvement and delay in time to definitive clinically meaningful deterioration in global health status/quality of life and breast symptoms favoring talazoparib versus chemotherapy (P < 0.01 for all), consistent with initial analyses.
Conclusions: In gBRCA1/2-mutated HER2-negative ABC, talazoparib did not significantly improve OS over chemotherapy; subsequent treatments may have impacted analysis. Safety was consistent with previous observations. PRO continued to favor talazoparib.
Keywords: PARP inhibitor; breast cancer; germline BRCA mutation; overall survival; talazoparib.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosures JKL reports grant or research support from Novartis, Pfizer, Genentech, GSK, EMD-Serono, AstraZeneca, and Zenith Epigenetics; fees for speakers' bureaus from Med Learning Group, Physician's Education Resource, Prime Oncology, Medscape, MedPage, Clinical Care Options, and UpToDate; honoraria from UpToDate; membership on advisory committees or review panels, or board membership, for AstraZeneca, Pfizer, and Ayala Pharmaceuticals (all uncompensated); membership on review panels for NCCN, ASCO, and NIH PDQ; patent, royalties, or other intellectual properties from UpToDate; travel, accommodation, and expenses from Med Learning Group, Physician's Education Resource, Medscape, and Clinical Care Options; and employment by the University of Texas MD Anderson Cancer Center. SAH reports contracted research support and editorial assistance from Ambrx, Amgen, Arvinas, Bayer, BioMarin, Cascadian Therapeutics, Daiichi-Sankyo, Dignitana, Genentech/Roche, GSK, Immunomedics, Lilly, MacroGenics, Merrimack, Novartis, Pfizer, OBI Pharma, Pieris Pharmaceuticals, Puma Biotechnology, Radius Health, Sanofi, and Seattle Genetics. LAM, HR, Y-HI, and WE have declared no conflicts of interest. K-HL reports honoraria from Roche and AstraZeneca and has participated in advisory boards for Bayer, Ono Pharmaceutical, Samsung Bioepis, Roche, Eisai, and AstraZeneca. HSR reports research support to the University of California San Francisco from Eisai, Genentech, Lilly, MacroGenics, Merck, Novartis, OBI Pharma, Odonate Therapeutics, Immunomedics, Daiichi-Sankyo, Seremonix, and Pfizer; a consulting role with Samsung and Puma; and travel support from Pfizer and Novartis. AG reports travel/accommodation/meeting registration fees from Pfizer, AstraZeneca, Roche, and Novartis. SD is a speaker and advisor for Pfizer, Novartis, Puma, Eli Lilly, Clovis, Genentech, AstraZeneca, Genomic Health, and Agendia. NW reports stock and ownership in CSL Behring, research funding (institution) from Medivation, honoraria for advisory boards from Novartis and Pfizer, and consultancy fees, honoraria for advisory boards and travel and accommodation fees from Roche. AG reports honoraria from AstraZeneca and Pfizer for participation in advisory boards. RY reports consulting fees from Roche, Pfizer, Novartis, and Eli Lilly, has been a speaker for Roche, Teva, Medison, MSD, AstraZeneca, Novartis, and Pfizer, and reports a grant from Roche. RGWQ was an employee of Pfizer when the study was carried out and reported ownership interest in Pfizer and Amgen. TU, SL, and AC are employees of Pfizer and own stocks in Pfizer. JLB reports consulting fees from Pfizer, Medivation, Amgen, Novartis, Genomic Health, Daiichi-Sankyo, and Myriad Genetics. MM reports research funding from Roche and Novartis and consulting or advisory role for Roche/Genentech, Novartis, AstraZeneca, Lilly, Taiho Pharmaceutical, PharmaMar, and Pfizer. JE has received consulting fees from Pfizer, Novartis, Lilly, Roche, and Tesaro; contracted research from Pfizer, Lilly, Novartis, Seattle Genetics, AstraZeneca, Roche, and Odonate; and travel support from AstraZeneca, Celgene, Pfizer, Novartis, Lilly, and Tesaro. Data sharing Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (i) for indications that have been approved in the US and/or EU or (ii) in programs that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Figures



Comment in
-
Survival benefits of PARP inhibitors in advanced breast cancer: a mirage?Ann Oncol. 2020 Nov;31(11):1432-1434. doi: 10.1016/j.annonc.2020.09.018. Epub 2020 Sep 29. Ann Oncol. 2020. PMID: 33007379 No abstract available.
-
PARP inhibitors coming of age.Nat Rev Clin Oncol. 2021 Feb;18(2):69-70. doi: 10.1038/s41571-020-00452-2. Nat Rev Clin Oncol. 2021. PMID: 33239729 No abstract available.
References
-
- Wang B, Chu D, Feng Y et al. Discovery and Characterization of (8S,9R)-5-Fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-2,7,8,9-te trahydro-3H-pyrido[4,3,2-de]phthalazin-3-one (BMN 673, Talazoparib), a Novel, Highly Potent, and Orally Efficacious Poly(ADP-ribose) Polymerase-1/2 Inhibitor, as an Anticancer Agent. J Med Chem 2016; 59: 335–357. - PubMed
-
- Turner NC, Telli ML, Rugo HS et al. A Phase II Study of Talazoparib After Platinum or Cytotoxic Nonplatinum Regimens in Patients With Advanced Breast Cancer and Germline BRCA1/2 Mutations (ABRAZO). Clin Cancer Res 2019; 25: 2717–2724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

