Efavirenz Pharmacogenetics and Weight Gain Following Switch to Integrase Inhibitor-Containing Regimens
- PMID: 32829410
- PMCID: PMC8492125
- DOI: 10.1093/cid/ciaa1219
Efavirenz Pharmacogenetics and Weight Gain Following Switch to Integrase Inhibitor-Containing Regimens
Abstract
Background: Unwanted weight gain affects some people living with human immunodeficiency virus (HIV) who are prescribed integrase strand transfer inhibitors (INSTIs). Mechanisms and risk factors are incompletely understood.
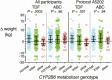
Methods: We utilized 2 cohorts to study pharmacogenetics of weight gain following switch from efavirenz- to INSTI-based regimens. In an observational cohort, we studied weight gain at 48 weeks following switch from efavirenz- to INSTI-based regimens among patients who had been virologically suppressed for at least 2 years at a clinic in the United States. Associations were characterized with CYP2B6 and UGT1A1 genotypes that affect efavirenz and INSTI metabolism, respectively. In a clinical trials cohort, we studied weight gain at 48 weeks among treatment-naive participants who were randomized to receive efavirenz-containing regimens in AIDS Clinical Trials Group studies A5095, A5142, and A5202 and did not receive INSTIs.
Results: In the observational cohort (n = 61), CYP2B6 slow metabolizers had greater weight gain after switch (P = .01). This was seen following switch to elvitegravir or raltegravir, but not dolutegravir. UGT1A1 genotype was not associated with weight gain. In the clinical trials cohort (n = 462), CYP2B6 slow metabolizers had lesser weight gain at week 48 among participants receiving efavirenz with tenofovir disoproxil fumarate (P = .001), but not those receiving efavirenz with abacavir (P = .65). Findings were consistent when stratified by race/ethnicity and by sex.
Conclusions: Among patients who switched from efavirenz- to INSTI-based therapy, CYP2B6 genotype was associated with weight gain, possibly reflecting withdrawal of the inhibitory effect of higher efavirenz concentrations on weight gain. The difference by concomitant nucleoside analogue is unexplained.
Trial registration: ClinicalTrials.gov NCT00013520 NCT00050895 NCT00118898.
Keywords: HIV-1; efavirenz; integrase strand transfer inhibitors; pharmacogenetics; weight gain.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures




References
-
- Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents.2018. Available at: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescent.... Accessed 20 June 2018.
-
- Raffi F, Rachlis A, Stellbrink HJ, et al. , SPRING-2 Study Group . Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 2013; 381:735–43. - PubMed
-
- Clotet B, Feinberg J, van Lunzen J, et al. , ING114915 Study Team . Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383:2222–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 AI027661/AI/NIAID NIH HHS/United States
- D43 TW010559/TW/FIC NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- R01 AI058740/AI/NIAID NIH HHS/United States
- UM1 TR004528/TR/NCATS NIH HHS/United States
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069503/AI/NIAID NIH HHS/United States
- UM1 AI069472/AI/NIAID NIH HHS/United States
- UM1 AI069513/AI/NIAID NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- UL1 TR001111/TR/NCATS NIH HHS/United States
- M01 RR000046/RR/NCRR NIH HHS/United States
- P30 DK048520/DK/NIDDK NIH HHS/United States
- R01 AI077505/AI/NIAID NIH HHS/United States
- UL1 TR002548/TR/NCATS NIH HHS/United States
- U54 AG062334/AG/NIA NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- UM1 AI069434/AI/NIAID NIH HHS/United States
- UL1 RR024996/RR/NCRR NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- UM1 AI069495/AI/NIAID NIH HHS/United States
- UM1 TR004406/TR/NCATS NIH HHS/United States
- I01 BX000207/BX/BLRD VA/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- U01 AI069439/AI/NIAID NIH HHS/United States
- UL1 TR002535/TR/NCATS NIH HHS/United States
- UM1 AI069428/AI/NIAID NIH HHS/United States
- K12 AR084234/AR/NIAMS NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- UL1 RR024156/RR/NCRR NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- UL1 TR002489/TR/NCATS NIH HHS/United States
- UM1 AI069415/AI/NIAID NIH HHS/United States
- UL1 TR000058/TR/NCATS NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI069484/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
- U01 AI046376/AI/NIAID NIH HHS/United States
- UM1 AI069481/AI/NIAID NIH HHS/United States
- T32 AI150547/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U01 AI046370/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- P30 AI073961/AI/NIAID NIH HHS/United States
- UM1 AI069474/AI/NIAID NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1 RR025777/RR/NCRR NIH HHS/United States
- U01 AI034853/AI/NIAID NIH HHS/United States
- K23 DK059311/DK/NIDDK NIH HHS/United States
- UM1 AI069452/AI/NIAID NIH HHS/United States
- UM1 AI069496/AI/NIAID NIH HHS/United States
- U01 AI027666/AI/NIAID NIH HHS/United States
- UL1 RR024160/RR/NCRR NIH HHS/United States
- U01 AI027658/AI/NIAID NIH HHS/United States
- UM1 AI069556/AI/NIAID NIH HHS/United States
- U01 AI027675/AI/NIAID NIH HHS/United States
- P30 AI110527/AI/NIAID NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
- UM1 TR004399/TR/NCATS NIH HHS/United States
- UL1 TR000170/TR/NCATS NIH HHS/United States
- UM1 AI069502/AI/NIAID NIH HHS/United States
- U01 AI025859/AI/NIAID NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- UM1 AI069450/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- A1069412/NH/NIH HHS/United States
- UL1 RR025747/RR/NCRR NIH HHS/United States
- UM1 AI069532/AI/NIAID NIH HHS/United States
- UM1 AI069465/AI/NIAID NIH HHS/United States
- U01 AI032782/AI/NIAID NIH HHS/United States
- K01 AI131895/AI/NIAID NIH HHS/United States
- UL1 RR025008/RR/NCRR NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- UL1 TR000457/TR/NCATS NIH HHS/United States
- UM1 AI069511/AI/NIAID NIH HHS/United States
- UM1 AI069447/AI/NIAID NIH HHS/United States
- UM1 AI069467/AI/NIAID NIH HHS/United States
- U01 HL146203/HL/NHLBI NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- UL1 TR003098/TR/NCATS NIH HHS/United States
- U01 AI025868/AI/NIAID NIH HHS/United States

