Pathophysiology of Diuretic Resistance and Its Implications for the Management of Chronic Heart Failure
- PMID: 32829662
- PMCID: PMC10683075
- DOI: 10.1161/HYPERTENSIONAHA.120.15205
Pathophysiology of Diuretic Resistance and Its Implications for the Management of Chronic Heart Failure
Abstract
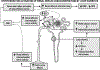
Diuretic resistance implies a failure to increase fluid and sodium (Na+) output sufficiently to relieve volume overload, edema, or congestion, despite escalating doses of a loop diuretic to a ceiling level (80 mg of furosemide once or twice daily or greater in those with reduced glomerular filtration rate or heart failure). It is a major cause of recurrent hospitalizations in patients with chronic heart failure and predicts death but is difficult to diagnose unequivocally. Pharmacokinetic mechanisms include the low and variable bioavailability of furosemide and the short duration of all loop diuretics that provides time for the kidneys to restore diuretic-induced Na+ losses between doses. Pathophysiological mechanisms of diuretic resistance include an inappropriately high daily salt intake that exceeds the acute diuretic-induced salt loss, hyponatremia or hypokalemic, hypochloremic metabolic alkalosis, and reflex activation of the renal nerves. Nephron mechanisms include tubular tolerance that can develop even during the time that the renal tubules are exposed to a single dose of diuretic, or enhanced reabsorption in the proximal tubule that limits delivery to the loop, or an adaptive increase in reabsorption in the downstream distal tubule and collecting ducts that offsets ongoing blockade of Na+ reabsorption in the loop of Henle. These provide rationales for novel strategies including the concurrent use of diuretics that block these nephron segments and even sequential nephron blockade with multiple diuretics and aquaretics combined in severely diuretic-resistant patients with heart failure.
Keywords: diuretics; edema; furosemide; heart failure; torsemide.
Figures


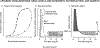



References
-
- ter Maaten JM, Valente MA, Damman K, Hillege HL, Navis G, Voors AA. Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nature reviews. Cardiology. 2015;12:184–192 - PubMed
-
- Testani JM, Hanberg JS, Cheng S, Rao V, Onyebeke C, Laur O, Kula A, Chen M, Wilson FP, Darlington A, Bellumkonda L, Jacoby D, Tang WH, Parikh CR. Rapid and highly accurate prediction of poor loop diuretic natriuretic response in patients with heart failure. Circulation. Heart failure. 2016;9:e002370 - PMC - PubMed
-
- Kiernan MS, Stevens SR, Tang WHW, Butler J, Anstrom KJ, Birati EY, Grodin JL, Gupta D, Margulies KB, LaRue S, Davila-Roman VG, Hernandez AF, de Las Fuentes L. Determinants of diuretic responsiveness and associated outcomes during acute heart failure hospitalization: An analysis from the nhlbi heart failure network clinical trials. Journal of cardiac failure. 2018;24:428–438 - PMC - PubMed
-
- Strobeck JE, Feldschuh J, Miller WL. Heart failure outcomes with volume-guided management. JACC. Heart failure. 2018;6:940–948 - PubMed
-
- Miller WL, Mullan BP. Understanding the heterogeneity in volume overload and fluid distribution in decompensated heart failure is key to optimal volume management: Role for blood volume quantitation. JACC. Heart failure. 2014;2:298–305 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

