On folding morphogenesis, a mechanical problem
- PMID: 32829686
- PMCID: PMC7482214
- DOI: 10.1098/rstb.2019.0564
On folding morphogenesis, a mechanical problem
Abstract
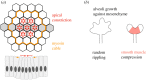
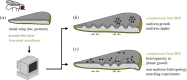
Tissue folding is a fundamental process that sculpts a simple flat epithelium into a complex three-dimensional organ structure. Whether it is the folding of the brain, or the looping of the gut, it has become clear that to generate an invagination or a fold of any form, mechanical asymmetries must exist in the epithelium. These mechanical asymmetries can be generated locally, involving just the invaginating cells and their immediate neighbours, or on a more global tissue-wide scale. Here, we review the different mechanical mechanisms that epithelia have adopted to generate folds, and how the use of precisely defined mathematical models has helped decipher which mechanisms are the key driving forces in different epithelia. This article is part of a discussion meeting issue 'Contemporary morphogenesis'.
Keywords: folding; mathematical models; mechanics; morphogenesis.
Conflict of interest statement
We have no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
