Cilostazol ameliorates diabetic nephropathy by inhibiting highglucose- induced apoptosis
- PMID: 32830147
- PMCID: PMC7445481
- DOI: 10.4196/kjpp.2020.24.5.403
Cilostazol ameliorates diabetic nephropathy by inhibiting highglucose- induced apoptosis
Abstract
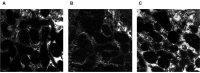
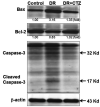
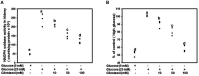
Diabetic nephropathy (DN) is a hyperglycemia-induced progressive development of renal insufficiency. Excessive glucose can increase mitochondrial reactive oxygen species (ROS) and induce cell damage, causing mitochondrial dysfunction. Our previous study indicated that cilostazol (CTZ) can reduce ROS levels and decelerate DN progression in streptozotocin (STZ)-induced type 1 diabetes. This study investigated the potential mechanisms of CTZ in rats with DN and in high glucose-treated mesangial cells. Male Sprague-Dawley rats were fed 5 mg/kg/day of CTZ after developing STZ-induced diabetes mellitus. Electron microscopy revealed that CTZ reduced the thickness of the glomerular basement membrane and improved mitochondrial morphology in mesangial cells of diabetic kidney. CTZ treatment reduced excessive kidney mitochondrial DNA copy numbers induced by hyperglycemia and interacted with the intrinsic pathway for regulating cell apoptosis as an antiapoptotic mechanism. In high-glucose-treated mesangial cells, CTZ reduced ROS production, altered the apoptotic status, and down-regulated transforming growth factor beta (TGF-β) and nuclear factor kappa light chain enhancer of activated B cells (NF-κB). Base on the results of our previous and current studies, CTZ deceleration of hyperglycemia-induced DN is attributable to ROS reduction and thereby maintenance of the mitochondrial function and reduction in TGF-β and NF-κB levels.
Keywords: Cilostazol; Diabetic nephropathy; Mesangial cell; Mitochondrial DNA; Oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Combination of leflunomide and benazepril reduces renal injury of diabetic nephropathy rats and inhibits high-glucose induced cell apoptosis through regulation of NF-κB, TGF-β and TRPC6.Ren Fail. 2019 Nov;41(1):899-906. doi: 10.1080/0886022X.2019.1665547. Ren Fail. 2019. PMID: 31552773 Free PMC article.
-
Andrographolide ameliorates diabetic nephropathy by attenuating hyperglycemia-mediated renal oxidative stress and inflammation via Akt/NF-κB pathway.Mol Cell Endocrinol. 2016 Dec 5;437:268-279. doi: 10.1016/j.mce.2016.06.029. Epub 2016 Jul 1. Mol Cell Endocrinol. 2016. PMID: 27378149
-
Cilostazol ameliorates nephropathy in type 1 diabetic rats involving improvement in oxidative stress and regulation of TGF-Beta and NF-kappaB.Biosci Biotechnol Biochem. 2010;74(7):1355-61. doi: 10.1271/bbb.90938. Epub 2010 Jul 7. Biosci Biotechnol Biochem. 2010. PMID: 20622454
-
Berberine ameliorates experimental diabetes-induced renal inflammation and fibronectin by inhibiting the activation of RhoA/ROCK signaling.Mol Cell Endocrinol. 2013 Dec 5;381(1-2):56-65. doi: 10.1016/j.mce.2013.07.019. Epub 2013 Jul 26. Mol Cell Endocrinol. 2013. PMID: 23896433
-
Reactive oxygen species-regulated signaling pathways in diabetic nephropathy.J Am Soc Nephrol. 2003 Aug;14(8 Suppl 3):S241-5. doi: 10.1097/01.asn.0000077410.66390.0f. J Am Soc Nephrol. 2003. PMID: 12874439 Review.
Cited by
-
Reno-protective effects of GLP-1 receptor agonist and anti-platelets in experimentally induced diabetic kidney disease in male albino rats.Iran J Basic Med Sci. 2022 Dec;25(12):1487-1497. doi: 10.22038/IJBMS.2022.65061.14494. Iran J Basic Med Sci. 2022. PMID: 36544522 Free PMC article.
-
Exposure to copper induces endoplasmic reticulum (ER) stress-mediated apoptosis in chicken (Gallus gallus) myocardium.Vet Res Commun. 2023 Dec;47(4):2027-2040. doi: 10.1007/s11259-023-10166-2. Epub 2023 Jul 5. Vet Res Commun. 2023. PMID: 37405676
-
The Role of Platelets in Diabetic Kidney Disease.Int J Mol Sci. 2022 Jul 27;23(15):8270. doi: 10.3390/ijms23158270. Int J Mol Sci. 2022. PMID: 35955405 Free PMC article. Review.
-
Exosomes as nanostructures deliver miR-204 in alleviation of mitochondrial dysfunction in diabetic nephropathy through suppressing methyltransferase-like 7A-mediated CIDEC N6-methyladenosine methylation.Aging (Albany NY). 2024 Feb 8;16(4):3302-3331. doi: 10.18632/aging.205535. Epub 2024 Feb 8. Aging (Albany NY). 2024. PMID: 38334961 Free PMC article.
-
Preclinical pharmacology and pharmacokinetics of curcumin tagged cilostazol nanodispersion for the management of diabetic nephropathy in wister rat model.In Silico Pharmacol. 2024 Sep 2;12(2):81. doi: 10.1007/s40203-024-00256-7. eCollection 2024. In Silico Pharmacol. 2024. PMID: 39233909 Free PMC article.
References
-
- Kitada M, Ogura Y, Suzuki T, Sen S, Lee SM, Kanasaki K, Kume S, Koya D. A very-low-protein diet ameliorates advanced diabetic nephropathy through autophagy induction by suppression of the mTORC1 pathway in Wistar fatty rats, an animal model of type 2 diabetes and obesity. Diabetologia. 2016;59:1307–1317. doi: 10.1007/s00125-016-3925-4. - DOI - PubMed
-
- Wang Z, do Carmo JM, Hall JE. ER stress and mitochondrial ROS contribute to the development of hypertensive‐diabetic nephropathy. FASEB J. 2016;30(Suppl 1):740.17.

