Curcumin targets vascular endothelial growth factor via activating the PI3K/Akt signaling pathway and improves brain hypoxic-ischemic injury in neonatal rats
- PMID: 32830149
- PMCID: PMC7445479
- DOI: 10.4196/kjpp.2020.24.5.423
Curcumin targets vascular endothelial growth factor via activating the PI3K/Akt signaling pathway and improves brain hypoxic-ischemic injury in neonatal rats
Abstract
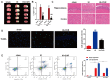
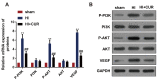
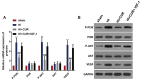
This study aimed to evaluate the effect of curcumin on brain hypoxicischemic (HI) damage in neonatal rats and whether the phosphoinositide 3-kinase (PI3K)/Akt/vascular endothelial growth factor (VEGF) signaling pathway is involved. Brain HI damage models were established in neonatal rats, which received the following treatments: curcumin by intraperitoneal injection before injury, insulin-like growth factor 1 (IGF-1) by subcutaneous injection after injury, and VEGF by intracerebroventricular injection after injury. This was followed by neurological evaluation, hemodynamic measurements, histopathological assessment, TUNEL assay, flow cytometry, and western blotting to assess the expression of p-PI3K, PI3K, p-Akt, Akt, and VEGF. Compared with rats that underwent sham operation, rats with brain HI damage showed remarkably increased neurological deficits, reduced right blood flow volume, elevated blood viscosity and haematocrit, and aggravated cell damage and apoptosis; these injuries were significantly improved by curcumin pretreatment. Meanwhile, brain HI damage induced the overexpression of p-PI3K, p-Akt, and VEGF, while curcumin pretreatment inhibited the expression of these proteins. In addition, IGF-1 treatment rescued the curcumin-induced down-regulated expression of p- PI3K, p-Akt, and VEGF, and VEGF overexpression counteracted the inhibitory effect of curcumin on brain HI damage. Overall, pretreatment with curcumin protected against brain HI damage by targeting VEGF via the PI3K/Akt signaling pathway in neonatal rats.
Keywords: Brain; Curcumin; Hypoxic-ischemic; PI3K/Akt signaling pathway; Vascular endothelial growth factor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

