Cloning of a Novel vpr Gene Encoding a Minor Fibrinolytic Enzyme from Bacillus subtilis SJ4 and the Properties of Vpr
- PMID: 32830189
- PMCID: PMC9728201
- DOI: 10.4014/jmb.2006.06014
Cloning of a Novel vpr Gene Encoding a Minor Fibrinolytic Enzyme from Bacillus subtilis SJ4 and the Properties of Vpr
Abstract
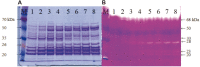
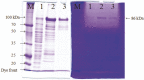
We have previously characterized AprESJ4, the major fibrinolytic enzyme from Bacillus subtilis SJ4 (Yao et al., 2019). During that study, we observed a 68 kDa protein with fibrinolytic activity. In this study, we cloned the gene (vprSJ4) encoding the 68 kDa protein, a mature Vpr and minor protease secreted by Bacillus species. vprSJ4 encodes a preproenzyme consisting of 810 amino acids (aa) including signal sequence (28 aa) and prosequence (132 aa). The mature enzyme (650 aa) has a predicted molecular weight of 68,467.35. Unlike Vprs from other B. subtilis strains, VprSJ4 has 4 additional amino acids (DEFA) at the C-terminus. vprSJ4 was overexpressed in Escherichia coli. PreproVprSJ4 was localized in inclusion bodies, and subjected to in vitro renaturation and purification by an affinity column. SDS-PAGE and western blot showed that autoprocessing of preproVprSJ4 occurred and 68 kDa and smaller proteins were produced. The optimum pH and temperature of the recombinant VprSJ4 were pH 7.0 and 40°C, respectively. Kinetic parameters of recombinant VprSJ4 were measured by using an artificial substrate, N-succinyl-ala-ala-pro-phe-p-nitroanilide. Coexpression of vprSJ4 and aprESJ4 using pHY300PLK increased the fibrinolytic activity a further 117% when compared with aprESJ4 single expression using the same vector in B. subtilis WB600.
Keywords: Bacillus subtilis; aprE; fibrinolytic activity; vpr.
Figures





Similar articles
-
Characterization of a Fibrinolytic Enzyme Secreted by Bacillus velezensis BS2 Isolated from Sea Squirt Jeotgal.J Microbiol Biotechnol. 2019 Mar 28;29(3):347-356. doi: 10.4014/jmb.1810.10053. J Microbiol Biotechnol. 2019. PMID: 30661324
-
Confirmation of Vpr as a fibrinolytic enzyme present in extracellular proteins of Bacillus subtilis.Protein Expr Purif. 2005 Jan;39(1):1-7. doi: 10.1016/j.pep.2004.08.008. Protein Expr Purif. 2005. PMID: 15596354
-
DNA Shuffling of aprE Genes to Increase Fibrinolytic Activity and Thermostability.J Microbiol Biotechnol. 2022 Jun 28;32(6):800-807. doi: 10.4014/jmb.2202.02017. Epub 2022 Apr 25. J Microbiol Biotechnol. 2022. PMID: 35484964 Free PMC article.
-
Characterization of a fibrinolytic enzyme secreted by Bacillus amyloliquefaciens CB1 and its gene cloning.J Microbiol Biotechnol. 2013;23(7):974-83. doi: 10.4014/jmb.1302.02065. J Microbiol Biotechnol. 2013. PMID: 23727811
-
Cloning and expression of a bpr gene encoding Bacillopeptidase F from Bacillus amyloliquefaciens CH86-1.J Microbiol Biotechnol. 2011 May;21(5):515-8. doi: 10.4014/jmb.1010.10061. J Microbiol Biotechnol. 2011. PMID: 21617349
Cited by
-
Auto- and Hetero-Catalytic Processing of the N-Terminal Propeptide Promotes the C-Terminal Fibronectin Type III Domain-Mediated Dimerization of a Thermostable Vpr-like Protease.Appl Environ Microbiol. 2022 Nov 8;88(21):e0150322. doi: 10.1128/aem.01503-22. Epub 2022 Oct 17. Appl Environ Microbiol. 2022. PMID: 36250702 Free PMC article.
References
-
- Choi NS, Chung DM, Park CS, Ahn KH, Kim JS, Song JJ, et al. Expression and identification of a minor extracellular fibrinolytic enzyme (Vpr) from Bacillus subtilis KCTC 3014. Biotechnol. Bioprocess Eng. 2010;15:446–452. doi: 10.1007/s12257-009-0191-z. - DOI

