Metabolism
- PMID: 32830223
- PMCID: PMC7545035
- DOI: 10.1042/EBC20190041
Metabolism
Abstract
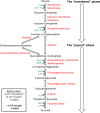
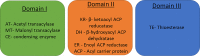
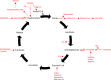
Metabolism consists of a series of reactions that occur within cells of living organisms to sustain life. The process of metabolism involves many interconnected cellular pathways to ultimately provide cells with the energy required to carry out their function. The importance and the evolutionary advantage of these pathways can be seen as many remain unchanged by animals, plants, fungi, and bacteria. In eukaryotes, the metabolic pathways occur within the cytosol and mitochondria of cells with the utilisation of glucose or fatty acids providing the majority of cellular energy in animals. Metabolism is organised into distinct metabolic pathways to either maximise the capture of energy or minimise its use. Metabolism can be split into a series of chemical reactions that comprise both the synthesis and degradation of complex macromolecules known as anabolism or catabolism, respectively. The basic principles of energy consumption and production are discussed, alongside the biochemical pathways that make up fundamental metabolic processes for life.
Keywords: biochemistry; glycolysis; metabolism.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures









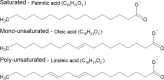










References
-
- Albrecht T. and Baron S. (1996) Medical Microbiology, University of Texas Medical Branch at Galveston, Dept. of Microbiology & Immunology, Galveston, TX, U.S.A., Chapter 4
-
- Berg J., Tymoczko J. and Stryer L. (2002) The first step in amino acid degradation is the removal of nitrogen. https://www.ncbi.nlm.nih.gov/books/NBK22475
-
- Brown G., Brown W. and Cohen P. (1962) Comparative biochemistry of urea synthesis IV. [14C]Urea synthesis by liver slices of the metamorphosing tadpole. Biochim. Biophys. Acta 60, 185–186 10.1016/0006-3002(62)90387-6 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

