Time-varying effects of 'optimized smoking treatment' on craving, negative affect and anhedonia
- PMID: 32830368
- PMCID: PMC7878324
- DOI: 10.1111/add.15232
Time-varying effects of 'optimized smoking treatment' on craving, negative affect and anhedonia
Abstract
Aims: To identify when smoking cessation treatments affect craving, negative affect and anhedonia, and how these symptoms relate to abstinence, to help evaluate the effects of particular intervention components in multi-component treatments and accelerate treatment refinement.
Design: Secondary analysis of data from a two-arm randomized controlled trial.
Setting: Seven primary care clinics in Wisconsin, United States.
Participants: Adult primary care patients who smoked daily (n = 574).
Intervention and comparator: Intervention was abstinence-optimized treatment (A-OT, n = 276) comprising 3 weeks of nicotine mini-lozenges pre-target quit day (TQD), 26 weeks of combination nicotine patch and mini-lozenges post-TQD and extensive psychosocial support. The comparator was recommended usual care (RUC, n = 298), comprising brief counseling and 8 weeks of nicotine patch post-TQD.
Measurements: Time-varying effect models examined dynamic effects of A-OT (versus RUC) on the primary outcomes of nightly cigarette craving, negative affect and anhedonia from 1 week pre- to 2 weeks post-TQD. Exploratory models examined within-person relations between nicotine medication use and same-day symptom ratings. Secondary logistic regression analyses examined associations between post-TQD craving, negative affect and anhedonia and 1-month post-TQD abstinence.
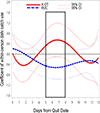
Findings: A-OT significantly suppressed pre- and post-TQD craving (β = -0.27 to -0.46 across days) and post-TQD anhedonia (β = -0.24 to -0.38 across days), relative to RUC. Within individuals, using patches was associated with lower negative affect in RUC (β = -0.42 to -0.52), but not in A-OT. Using more mini-lozenges was associated with greater craving (β = 0.04-0.07) and negative affect (β = 0.03-0.05) early, and with lower anhedonia (β = -0.06 to -0.12) later. Greater post-TQD craving (OR = 0.68) and anhedonia (OR = 0.85) predicted lower odds of abstinence 1 month post-TQD.
Conclusion: Time-varying effect models showed that a multi-component treatment intervention for smoking cessation suppressed significant withdrawal symptoms more effectively than recommended usual care among daily adult smokers motivated to quit. The intervention reduced craving pre- and post-target quit day (TQD) and anhedonia post-TQD.
Keywords: Multi-phase optimization strategy; nicotine replacement therapy; smoking cessation; time-varying effect modeling; treatment refinement; withdrawal.
© 2020 Society for the Study of Addiction.
Conflict of interest statement
Figures

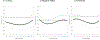

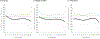
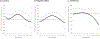
Similar articles
-
Comparative effects of varenicline or combination nicotine replacement therapy versus patch monotherapy on candidate mediators of early abstinence in a smoking cessation attempt.Addiction. 2021 Apr;116(4):926-935. doi: 10.1111/add.15248. Epub 2020 Oct 1. Addiction. 2021. PMID: 32888230 Free PMC article. Clinical Trial.
-
Anhedonia: Its Dynamic Relations With Craving, Negative Affect, and Treatment During a Quit Smoking Attempt.Nicotine Tob Res. 2017 Jun 1;19(6):703-709. doi: 10.1093/ntr/ntw247. Nicotine Tob Res. 2017. PMID: 28486709 Free PMC article. Clinical Trial.
-
What a difference a day makes: differences in initial abstinence response during a smoking cessation attempt.Addiction. 2017 Feb;112(2):330-339. doi: 10.1111/add.13613. Epub 2016 Oct 28. Addiction. 2017. PMID: 27633341 Free PMC article. Clinical Trial.
-
Progressive nicotine patch dosing prior to quitting smoking: feasibility, safety and effects during the pre-quit and post-quit periods.Addiction. 2019 Mar;114(3):515-522. doi: 10.1111/add.14483. Epub 2018 Dec 11. Addiction. 2019. PMID: 30370685
-
[Smoking reduction and temporary abstinence: new approaches for smoking cessation].J Mal Vasc. 2003 Dec;28(5):293-300. J Mal Vasc. 2003. PMID: 14978435 Review. French.
Cited by
-
Exploring the association between anhedonia and nicotine dependence: A study among female undergraduate students in Saudi Arabia.Tob Induc Dis. 2025 Apr 30;23. doi: 10.18332/tid/203551. eCollection 2025. Tob Induc Dis. 2025. PMID: 40309026 Free PMC article.
-
Time-Varying Mediation of Pharmacological Smoking Cessation Treatments on Smoking Lapse via Craving, Cessation Fatigue, and Negative Mood.Nicotine Tob Res. 2022 Oct 17;24(10):1548-1555. doi: 10.1093/ntr/ntac068. Nicotine Tob Res. 2022. PMID: 35287166 Free PMC article. Clinical Trial.
-
Effectiveness of smoking cessation on the high-risk population of lung cancer with early screening: a systematic review and meta-analysis of randomized controlled trials until January 2022.Arch Public Health. 2023 Jun 3;81(1):101. doi: 10.1186/s13690-023-01111-5. Arch Public Health. 2023. PMID: 37268972 Free PMC article.
-
Dyadic Synchrony and Responsiveness Within the Context of Elevated Autism Likelihood: Applying Time-Varying Effect Models.J Autism Dev Disord. 2025 Jun 11. doi: 10.1007/s10803-025-06891-z. Online ahead of print. J Autism Dev Disord. 2025. PMID: 40500588
-
Expanding the scope of the withdrawal syndrome: Anhedonia as a core nicotine withdrawal symptom.J Psychopathol Clin Sci. 2025 Jul;134(5):540-553. doi: 10.1037/abn0000981. Epub 2025 Apr 7. J Psychopathol Clin Sci. 2025. PMID: 40193440
References
-
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

