Metal-Templated Design of Chemically Switchable Protein Assemblies with High-Affinity Coordination Sites
- PMID: 32830423
- PMCID: PMC7983065
- DOI: 10.1002/anie.202009226
Metal-Templated Design of Chemically Switchable Protein Assemblies with High-Affinity Coordination Sites
Abstract
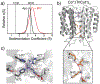
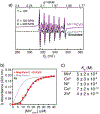
To mimic a hypothetical pathway for protein evolution, we previously tailored a monomeric protein (cyt cb562 ) for metal-mediated self-assembly, followed by re-design of the resulting oligomers for enhanced stability and metal-based functions. We show that a single hydrophobic mutation on the cyt cb562 surface drastically alters the outcome of metal-directed oligomerization to yield a new trimeric architecture, (TriCyt1)3. This nascent trimer was redesigned into second and third-generation variants (TriCyt2)3 and (TriCyt3)3 with increased structural stability and preorganization for metal coordination. The three TriCyt variants combined furnish a unique platform to 1) provide tunable coupling between protein quaternary structure and metal coordination, 2) enable the construction of metal/pH-switchable protein oligomerization motifs, and 3) generate a robust metal coordination site that can coordinate all mid-to-late first-row transition-metal ions with high affinity.
Keywords: bioinorganic chemistry; metalloproteins; protein design; protein structures; supramolecular chemistry.
© 2020 Wiley-VCH GmbH.
Figures


Similar articles
-
Design and Construction of Functional Supramolecular Metalloprotein Assemblies.Acc Chem Res. 2019 Feb 19;52(2):345-355. doi: 10.1021/acs.accounts.8b00617. Epub 2019 Jan 30. Acc Chem Res. 2019. PMID: 30698941
-
Metal-Directed Design of Supramolecular Protein Assemblies.Methods Enzymol. 2016;580:223-50. doi: 10.1016/bs.mie.2016.05.009. Epub 2016 Jun 24. Methods Enzymol. 2016. PMID: 27586336 Free PMC article.
-
Metal-mediated self-assembly of protein superstructures: influence of secondary interactions on protein oligomerization and aggregation.J Am Chem Soc. 2008 May 14;130(19):6082-4. doi: 10.1021/ja8012177. Epub 2008 Apr 19. J Am Chem Soc. 2008. PMID: 18422313 Free PMC article.
-
Functionalization of protein crystals with metal ions, complexes and nanoparticles.Curr Opin Chem Biol. 2018 Apr;43:68-76. doi: 10.1016/j.cbpa.2017.11.015. Epub 2017 Dec 12. Curr Opin Chem Biol. 2018. PMID: 29245143 Review.
-
Metal-directed protein self-assembly.Acc Chem Res. 2010 May 18;43(5):661-72. doi: 10.1021/ar900273t. Acc Chem Res. 2010. PMID: 20192262 Free PMC article. Review.
Cited by
-
Computationally Guided Redesign of a Heme-free Cytochrome with Native-like Structure and Stability.Biochemistry. 2022 Oct 4;61(19):2063-2072. doi: 10.1021/acs.biochem.2c00369. Epub 2022 Sep 15. Biochemistry. 2022. PMID: 36106943 Free PMC article.
-
Design Principles Of Inorganic-Protein Hybrid Materials for Biomedicine.Exploration (Beijing). 2025 Mar 6;5(3):20240182. doi: 10.1002/EXP.20240182. eCollection 2025 Jun. Exploration (Beijing). 2025. PMID: 40585774 Free PMC article. Review.
-
Overcoming universal restrictions on metal selectivity by protein design.Nature. 2022 Mar;603(7901):522-527. doi: 10.1038/s41586-022-04469-8. Epub 2022 Mar 2. Nature. 2022. PMID: 35236987 Free PMC article.
-
Protein Assembly by Design.Chem Rev. 2021 Nov 24;121(22):13701-13796. doi: 10.1021/acs.chemrev.1c00308. Epub 2021 Aug 18. Chem Rev. 2021. PMID: 34405992 Free PMC article. Review.
-
Genetically Engineered Liposwitch-Based Nanomaterials.Biomacromolecules. 2024 Dec 9;25(12):8058-8068. doi: 10.1021/acs.biomac.4c01388. Epub 2024 Nov 4. Biomacromolecules. 2024. PMID: 39495202 Free PMC article.
References
-
- Waldron KJ, Rutherford JC, Ford D, Robinson NJ, Nature 2009, 460, 823–830; - PubMed
- Williams RJP, J. Chem. Soc., Dalton Trans 1991, 539‒546;
- Frausto da Silva JJR, Williams RJP, The biological chemistry of the elements, Oxford University Press, Oxford, 2001.
-
- Bertini I, Gray HB, Stiefel EI, Valentine JS, Biological Inorganic Chemistry, Structure & Reactivity, University Science Books, Sausalito, 2007;
- Lippard S, Berg J, Principles of Bioinorganic Chemistry, University Science Books, Mill Valley, 1994;
- Holm RH, Kennepohl P, Solomon EI, Chem. Rev 1996, 96, 2239–2314; - PubMed
- Holm RH, Solomon EI, 2014, 114, 3367–3368. - PubMed
-
- Lu Y, Yeung N, Sieracki N, Marshall NM, Nature 2009, 460, 855–862; - PMC - PubMed
- Yu F, Cangelosi VM, Zastrow ML, Tegoni M, Plegaria JS, Tebo AG, Mocny CS, Ruckthong L, Qayyum H, Pecoraro VL, Chem. Rev 2014, 114, 3495–3578; - PMC - PubMed
- Schwizer F, Okamoto Y, Heinisch T, Gu Y, Pellizzoni MM, Lebrun V, Reuter R, Köhler V, Lewis JC, Ward TR, Chem. Rev 2018, 118, 142–231. - PubMed

