Molecular diagnostic challenges for non-retinal developmental eye disorders in the United Kingdom
- PMID: 32830442
- PMCID: PMC8432170
- DOI: 10.1002/ajmg.c.31837
Molecular diagnostic challenges for non-retinal developmental eye disorders in the United Kingdom
Abstract
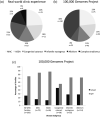
Overall, approximately one-quarter of patients with genetic eye diseases will receive a molecular diagnosis. Patients with developmental eye disorders face a number of diagnostic challenges including phenotypic heterogeneity with significant asymmetry, coexisting ocular and systemic disease, limited understanding of human eye development and the associated genetic repertoire, and lack of access to next generation sequencing as regarded not to impact on patient outcomes/management with cost implications. Herein, we report our real world experience from a pediatric ocular genetics service over a 12 month period with 72 consecutive patients from 62 families, and that from a cohort of 322 patients undergoing whole genome sequencing (WGS) through the Genomics England 100,000 Genomes Project; encompassing microphthalmia, anophthalmia, ocular coloboma (MAC), anterior segment dysgenesis anomalies (ASDA), primary congenital glaucoma, congenital cataract, infantile nystagmus, and albinism. Overall molecular diagnostic rates reached 24.9% for those recruited to the 100,000 Genomes Project (73/293 families were solved), but up to 33.9% in the clinic setting (20/59 families). WGS was able to improve genetic diagnosis for MAC patients (15.7%), but not for ASDA (15.0%) and congenital cataracts (44.7%). Increased sample sizes and accurate human phenotype ontology (HPO) terms are required to improve diagnostic accuracy. The significant mixed complex ocular phenotypes distort these rates and lead to missed variants if the correct gene panel is not applied. Increased molecular diagnoses will help to explain the genotype-phenotype relationships of these developmental eye disorders. In turn, this will lead to improved integrated care pathways, understanding of disease, and future therapeutic development.
Keywords: developmental eye disorders; genetic eye disease; next generation sequencing; targeted gene panels; whole genome sequencing.
© 2020 The Authors. American Journal of Medical Genetics Part C: Seminars in Medical Genetics published by Wiley Periodicals LLC.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Audo, I., Bujakowska, K. M., Leveillard, T., Mohand‐Said, S., Lancelot, M. E., Germain, A., … Zeitz, C. (2012). Development and application of a next‐generation‐sequencing (NGS) approach to detect known and novel gene defects underlying retinal diseases. Orphanet Journal of Rare Diseases, 7, 8. 10.1186/1750-1172-7-8 - DOI - PMC - PubMed
-
- Bernardis, I., Chiesi, L., Tenedini, E., Artuso, L., Percesepe, A., Artusi, V., … Tagliafico, E. (2016). Unravelling the complexity of inherited retinal dystrophies molecular testing: Added value of targeted next‐generation sequencing. BioMed Research International, 2016, 6341870–6341814. 10.1155/2016/6341870 - DOI - PMC - PubMed
-
- Capasso, J. (2014). The cost of genetic testing for ocular disease: Who pays? Current Opinion in Ophthalmology, 25, 394–399. - PubMed

